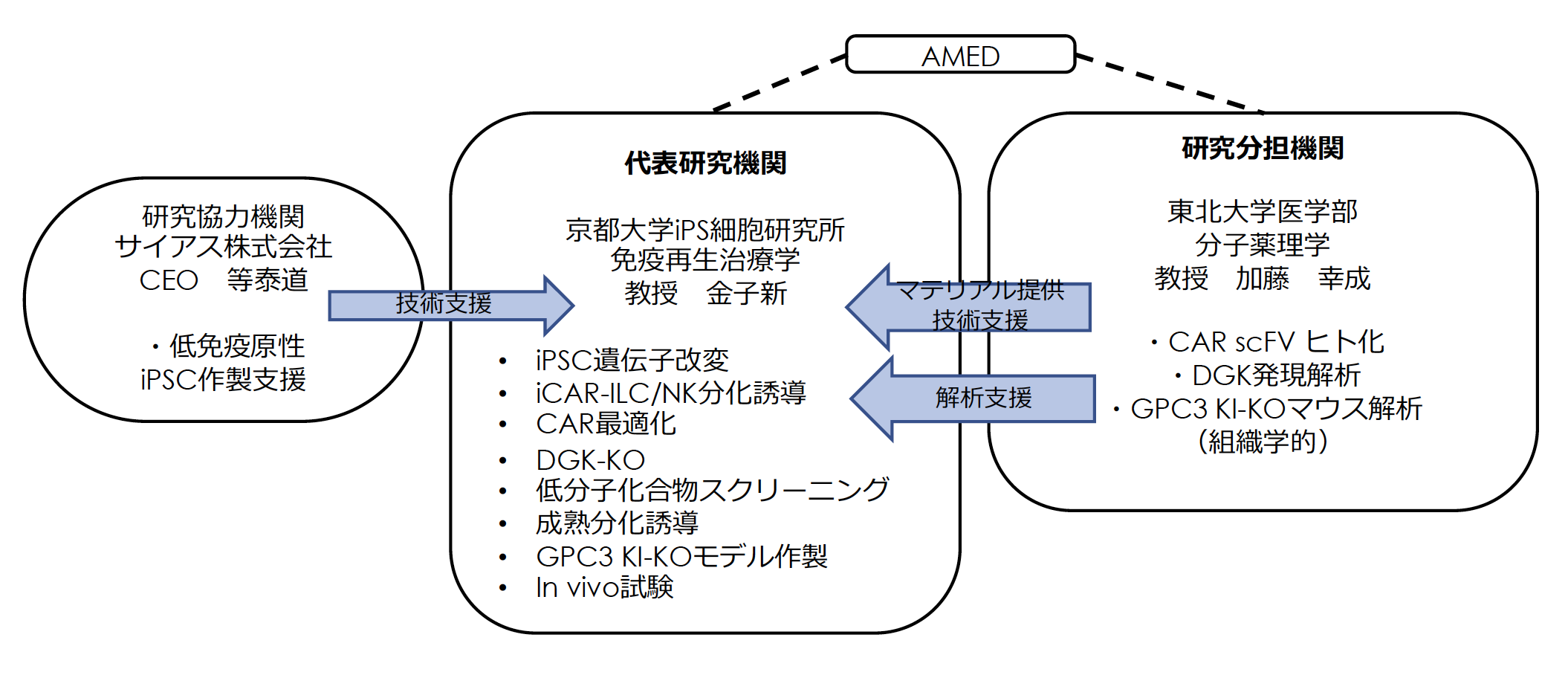
再生・細胞医療・遺伝子治療実現加速化プログラム
再生医療(AMED) 分担研究者:東北大学 加藤幸成(代表:京都大学 金子 新 )
<文献>
2025
Yamamoto H, Suzuki H, Tanaka T, Satofuka H, Kaneko MK, Kato Y. Microbes & Immunity , 3(1), 172–180; https://doi.org/10.36922/MI025130028, 2026 (PDF ; preprint )
Ubukata R, Suzuki H, Kaneko MK, Kato Y. Biochem Biophys Rep , 45, 102401, https://www.sciencedirect.com/science/article/pii/S2405580825004881, 2026 (PDF ; preprint )
VIDEO
Ohishi T, Suzuki H, Kaneko MK, Tanaka T, Harakawa A, Yoshida J, Tatsuda D, Kato Y, Kawada M. Cancer Sci , 116(12):3417-3430, doi: 10.1111/cas.70198, 2025 (PDF ; preprint )
VIDEO
Tanaka T, Yamamoto H, Kaneko Y, Shinoda K, Nakamura T, Li G, Fujisawa S, Satofuka H, Kaneko MK, Suzuki H, Kato Y. Microbes & Immunity , 2(4), 150–160; https://doi.org/10.36922/MI025060010, 2025 (PDF ; preprint )
VIDEO
Okada A, Suzuki H, Tanaka T, Kaneko MK, Kato Y. Biochem Biophys Rep , 44, 102265, https://doi.org/10.1016/j.bbrep.2025.102265, 2025 (PDF ; preprint )
Ubukata R, Ohishi T, Kaneko MK, Suzuki H, Kato Y. Int. J. Mol. Sci. , 26(17), 8302; https://doi.org/10.3390/ijms26178302, 2025 (PDF ; preprint )
Suzuki H, Ohishi T, Nakamura T, Yanaka M, Handa S, Tanaka T, Kaneko MK, Kato Y. antibodies , 14(3), 67; https://doi.org/10.3390/antib14030067, 2025 (PDF ; preprint )
Ubukata R, Suzuki H, Hirose M, Satofuka H, Tanaka T, Kaneko MK, Kato Y. Microbes & Immunity , 2(3), 168–177, https://doi.org/10.36922/mi.5728, 2025 (PDF ; preprint )
VIDEO
Haruto Yamamoto, Hiroyuki Suzuki, Tomohiro Tanaka, Mika K. Kaneko, Yukinari Kato Biochem Biophys Rep , 43, 102170, https://doi.org/10.1016/j.bbrep.2025.102170, 2025 (PDF ; preprint )
Ubukata R, Suzuki H, Tanaka T, Kaneko MK, Kato Y. Biochem Biophys Rep , 43, 1021382025, https://doi.org/10.1016/j.bbrep.2025.102138, 2025 (PDF ; preprint )
Kaneko Y, Tanaka T, Fujisawa S, Li G, Satofuka H, Kaneko MK, Suzuki H, Kato Y. PDF ; preprint )
VIDEO
Satofuka H, Suzuki H, Hirose M, Shinoda K, Nakamura T, Tanaka T, Kaneko MK, Kato Y. Biochem Biophys Rep , 43, 102130, https://www.sciencedirect.com/science/article/pii/S2405580825002171, 2025 (PDF ; preprint )
Tanaka T, Suzuki H, Taruta H, Saga A, Li G, Fujisawa S, Kaneko MK, Kato Y. Monoclon. Antib. Immunodiagn. Immunother. , 44(3), 41-52, DOI: 10.1089/mab.2025.0006, 2025 (PDF ; preprint )
Yamamoto H, Kaneko Y, Tanaka T, Li G, Suzuki H, Kaneko MK, Kato Y. Microbes & Immunity , 2(2), 126–136, https://doi.org/10.36922/mi.4661, 2025 (PDF ; preprint )
Satofuka H, Suzuki H, Tanaka T, Li G, Kaneko MK, Kato Y. Biochem Biophys Rep , 42, 1019982024, 2025 (PDF ; preprint )
VIDEO
Li G, Suzuki H, Tanaka T, Satofuka H, Kaneko MK, Kato Y. Biochem Biophys Rep , 42, 101965, 2025 (PDF ; preprint )
VIDEO
Tanaka T, Kaneko Y, Yamamoto H, Li G, Fujisawa S, Satofuka H, Shinoda K, Nakamura T, Kaneko MK, Suzuki H, Kato Y. Biochem Biophys Rep , 41, 101960, https://doi.org/10.1016/j.bbrep.2025.101960, 2024 (PDF ; preprint )
Satofuka H, Suzuki H, Tanaka T, Ubukata R, Hirose M, Yamamoto H, Kaneko Y, Fujisawa S, Li G, Kaneko MK, Kato Y. Biochem Biophys Rep , 41, 101948, https://doi.org/10.1016/j.bbrep.2025.101948, 2025 (PDF ; preprint )
VIDEO
Ishikawa K, Suzuki H,Tanaka T,Kaneko MK, Kato Y. MI , 2(1), 101–113; https://doi.org/10.36922/mi.5664, 2025 (PDF ; preprint )
VIDEO
Kaneko MK, Suzuki H, Ohishi T, Nakamura T, Yanaka M, Tanaka T, Kato Y. Int. J. Mol. Sci. , 2025(PDF ; preprint )
VIDEO
Mishima Y, Okada S, Ishikawa A, Wang B, Waseda M, Kaneko MK, Kato Y, Kaneko S. PDF )
2024
Tanaka T, Suzuki H, Ohishi T, Kaneko MK, Kato Y. PDF ; preprint )VIDEO
Hirose M, Suzuki H, Ubukata R, Tanaka T, Kaneko MK, Kato Y. Biochem Biophys Rep , 40, 101824, https://doi.org/10.1016/j.bbrep.2024.101824, 2024 (PDF ; preprint )
VIDEO
Ishikawa K, Suzuki H, Ohishi T, Nakamura T, Yanaka M, Li G, Tanaka T, Kawada M, Kaneko MK, Ohkoshi A, Katori Y, Kato Y.
Oncology reports , 52(5), 147, https://doi.org/10.3892/or.2024.8806, (PDF ; preprint )VIDEO
Ishikawa K, Suzuki H, Ohishi T, Li G, Tanaka T, Kawada M, Ohkoshi A, Kaneko MK, Katori Y, Kato Y. Int. J. Mol. Sci. , 25(17), 9190; https://doi.org/10.3390/ijms25179190, 2024 (PDF ; preprint )
VIDEO
Ubukata R, Suzuki H, Tanaka T, Li G, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(4), 112-118, https://doi.org/10.1089/mab.2024.0009, 2024 (PDF )
Suzuki H, Tanaka T, Li G, Ouchida T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(4), 96-100, https://doi.org/10.1089/mab.2024.0004, 2024 (PDF ; preprint )
Kobayashi H, Suzuki H, Tanaka T, Kaneko MK, Kato Y. Monoclon. Antib. Immunodiagn. Immunother. , 43(4), 101-107, https://doi.org/10.1089/mab.2024.0002, 2024 (PDF ; preprint )
Suzuki H, Ohishi T, Tanaka T, Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 25(15), 8386; https://doi.org/10.3390/ijms25158386, 2024 (PDF ; preprint )
VIDEO
Arimori T, Mihara E, Suzuki H, Ohishi T, Tanaka T, Kaneko MK, Takagi J, Kato Y.
Structure , 32(5),536-549, https://doi.org/10.1016/j.str.2024.02.007, 2024 (PDF )
VIDEO
Li G, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 59-66, DOI: 10.20944/preprints202311.0501.v2, 2023 (PDF ;preprint )
Ouchida T, Li G, Suzuki H, Yanaka M, Nakamura T, Handa S, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 53-58, doi:10.1089/mab.2024.0003, 2024 (PDF )
Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 35-43,
DOI: 10.1089/mab.2023.0033, 2024 (PDF ;online ;preprint )
Ouchida T, Isoda Y, Nakamura T, Yanaka M, Tanaka T, Handa S, Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 67-74, https://doi.org/10.1089/mab.2023.0032, 2024 (PDF ;preprint )
Okada Y, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 44-52, https://doi.org/10.1089/mab.2023.0029, 2024 (PDF ;preprint )
Inoue T, Yamamoto Y, Sato K, Nakamura Y, Shimizu Y, Ogawa M, Onodera T, Takahashi Y, Wakita T, Kaneko MK, Fukasawa M, Kato Y, Noguchi K.
iScience ,27(4),109363, DOI:https://doi.org/10.1016/j.isci.2024.109363, 2024 (PDF )
Ouchida T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(1), 17–23, https://doi.org/10.1089/mab.2023.0014, 2024 (PDF ; preprint )
Okada Y, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(1), 24–31, https://doi.org/10.1089/mab.2023.0016, 2024 (PDF ; preprint )
Ouchida T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(1), 10–16, DOI: 10.1089/mab.2023.0023, 2024 (PDF ;preprint )
Kaneko MK, Suzuki H, Ohishi T, Nakamura T, Tanaka T, Kato Y.
Int. J. Mol. Sci. , 25(3), 1941; https://doi.org/10.3390/ijms25031941, 2024 (PDF )
Tanaka T, Suzuki H, Ohishi T, Kaneko MK, Kato Y.
Cancer Sci. , 115(1), 298-309, https://doi.org/10.1111/cas.16008, 2024 (PDF ; preprint )
Suzuki H, Ohishi T, Tanaka T Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 25, 161. https://doi.org/10.3390/ijms25010161, 2024 (PDF ; preprint )
2023
Isoda Y, Kaneko MK, Tanaka T, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(6), 189–193, DOI: 10.1089/mab.2023.0026, 2023 (PDF ; preprint )
Ouchida T, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(6), 209–215, https://doi.org/10.1089/mab.2023.0020, 2023 (PDF ; preprint )
Suzuki H, Tanaka T, Kudo Y, Tawara M, Hirayama A, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(6), 203–208, DOI: 10.1089/mab.2023.0018, 2023 (PDF ; preprint )
Nanamiya R, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,42(5), 153–156, https://doi.org/10.1089/mab.2023.0015, 2023 (PDF ; preprint )
Suzuki H, Ohishi T, Kaneko MK, Kato Y.
Cancers , 15(20), 5080; https://doi.org/10.3390/cancers15205080, 2023 (PDF )
Suzuki H, Ohishi T, Nanamiya R, Kawada M, Kaneko MK, Kato Y.
Curr. Issues Mol. Biol. , 45(10), 7734-7748; https://doi.org/10.3390/cimb45100488, 2023 (PDF )
Ouchida T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Int. J. Transl. Med. , 3(3), 310-320; 2023 (PDF )
動画(YouTube)
総説(pdf)
AMED project
再生医療実現拠点ネットワークプログラム
再生医療(AMED) 分担研究者:東北大学 加藤幸成(代表:京都大学 金子 新 )
<文献>
2023
Suzuki H, Ohishi T, Nanamiya R, Kawada M, Kaneko MK, Kato Y.
Curr. Issues Mol. Biol. , 45(10), 7734-7748; https://doi.org/10.3390/cimb45100488, 2023 (PDF )
Suzuki H, Goto N, Tanaka T, Ouchida T, Kaneko MK, Kato Y.
antibodies , 12(3), 45; https://doi.org/10.3390/antib12030045, 2023 (PDF )
Suzuki H, Kitamura K, Goto N, Ishikawa K, Ouchida T, Tanaka T, Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 24(9), 8411; https://doi.org/10.3390/ijms24098411, 2023 (PDF )
Kudo Y, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
antibodies , 12(2), 31, https://doi.org/10.3390/antib12020031, 2023 (PDF )
Isoda Y, Tanaka Y, Suzuki H, Asano T, Kitamura K, Kudo Y, Ejima R, Ozawa K, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(2), 73–76, https://doi.org/10.1089/mab.2022.0035, 2023 (PDF )
Tateyama N, Asano T, Tanaka T, Isoda T, Okada Y, Kobayashi H, Li G, Nanamiya R, Yoshikawa T, Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(2), 68–72, https://doi.org/10.1089/mab.2022.0034, 2023 (PDF )
Suzuki H, Ozawa K, Tanaka T, Kaneko MK, Kato Y.
Biomedicines , 11(4), 1099; https://doi.org/10.3390/biomedicines11041099, 2023 (PDF )
Li G, Suzuki H, Tanaka T, Asano T, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 41–47, doi: 10.1089/mab.2022.0031, 2023 (PDF )
Kobayashi H, Asano T, Suzuki H, Tanaka T, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 15–21, https://doi.org/10.1089/mab.2022.0032, 2022 (PDF )
Nanamiya R, Ohishi T, Suzuki H, Mizuno T, Yoshikawa T, Asano T, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 27–33, doi:10.1089/mab.2022.0022, 2022 (PDF )
Suzuki H, Asano T, Ohishi T, Yoshikawa T, Suzuki H, Mizuno T, Tanaka T, Kawada M, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 34–40, doi: 10.1089/mab.2022.0023, 2022 (PDF )
Isoda Y, Tanaka T, Suzuki H, Asano T, Yoshikawa T, Kitamura K, Kudo Y, Ejima R, Ozawa K, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 22–26, doi: 10.1089/mab.2022.0029, 2022 (PDF )
Suzuki H, Tanaka T, Goto N, Kaneko MK, Kato Y.
Curr. Issues Mol. Biol. , 45(3), 1875-1888; https://doi.org/10.3390/cimb45030121, 2023 (PDF )
Ejima R, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 24(4), 4007; https://doi.org/10.3390/ijms24044007, 2023 (PDF )
Kobayashi H, Asano T, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
antibodies , 12(1), 11; https://doi.org/10.3390/antib12010011, 2023 (PDF )
29. Li G, Suzuki H, Ohishi T, Asano T, Tanaka T, Yanaka M, Nakamura T, Yoshikawa T, Kawada M, Kaneko MK, Kato Y.
Int J Mol Med , 51(2), 18, https://doi.org/10.3892/ijmm.2023.5221, 2023 (PDF )
28. Ohishi T, Kaneko MK, Yoshida Y, Takashima A, Kato Y, Kawada M.
Int. J. Mol. Sci. , 24(2), 1702; https://doi.org/10.3390/ijms24021702, 2023 (PDF )
2022
Asano T, Tanaka T, Suzuki H, Li G, Nanamiya R, Tateyama N, Isoda Y, Okada Y, Kobayashi H, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 343–349, doi: 10.1089/mab.2022.0021, 2022 (PDF )
Tanaka T, Suzuki H, Asano T, Li G, Nanamiya R, Tateyama N, Isoda Y, Okada Y, Kobayashi H, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 339–342, doi:10.1089/mab.2022.0020, 2022 (PDF )
Saito M, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 333–338, https://doi.org/10.1089/mab.2021.0069, 2022 (PDF )
Kawabata H, Ohishi T, Suzuki H, Asano T, Kawada M, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 320–327, https://doi.org/10.1089/mab.2021.0049, 2022 (PDF )
Nanamiya R, Suzuki H, Takei J, Li G, Goto N, Harada H, Saito M, Tanaka T, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 311–319, https://doi.org/10.1089/mab.2021.0058, 2022 (PDF )
Tanaka T, Suzuki H, Isoda Y, Asano T, Nakamura T, Yanaka M, Handa S, Takahashi N, Okuno S, Yoshikawa T, Li G, Nanamiya R, Goto N, Tateyama N, Okada Y, Kobayashi H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 303–310, doi: 10.1089/mab.2022.0027, 2022 (PDF )
Tateyama N, Asano T, Suzuki H, Li G, Yoshikawa T, Tanaka T, Kaneko MK, Kato Y.
antibodies , 11(4), 75; https://doi.org/10.3390/antib11040075, 2022 (PDF )
Asano T, Tanaka T, Suzuki H, Li G, Ohishi T, Kawada M, Yoshikawa T, Kaneko MK, Kato Y.
antibodies , 11(4), 74; https://doi.org/10.3390/antib11040074, 2022 (PDF )
Tateyama N, Suzuki H, Ohishi T, Asano T, Tanaka T, Mizuno T, Yoshikawa T, Kawada M, Kaneko MK, Kato Y.
pharmaceutics ,14(11), 2494; https://doi.org/10.3390/pharmaceutics14112494, 2022 (PDF )
Isoda Y, Tanaka T, Suzuki H, Asano T, Nakamura T, Yanaka M, Handa S, Komatsu Y, Okuno S, Takahashi N, Okada Y, Kobayashi H, Li G, Nanamiya R, Goto N, Tateyama N, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,41(5),275–278, DOI: 10.1089/mab.2022.0019, 2022 (PDF )
Tanaka T, Suzuki H, Li G, Nanamiya R, Isoda Y, Okada Y, Kobayashi H, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,41(5),285–289, DOI: 10.1089/mab.2022.0018, 2022 (PDF )
Saito M, Suzuki H, Asano T, Tanaka T, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,41(5),279–284, https://doi.org/10.1089/mab.2022.0016, 2022 (PDF )
Ishikawa A, Waseda M, Ishii T, Kaneko MK, Kato Y, Kaneko S.
Genes Cells, 27(9), 549-558, https://doi.org/10.1111/gtc.12972, 2022 (PDF )
Tanaka T, Li G, Saito M, Suzuki H, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 188–193, https://doi.org/10.1089/mab.2022.0001, 2022 (PDF )
Kudo Y, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 194–201, https://doi.org/10.1089/mab.2022.0007, 2022 (PDF )
Asano T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 214–220, https://doi.org/10.1089/mab.2022.0015, 2022 (PDF )
Okada Y, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 221–227, https://doi.org/10.1089/mab.2022.0017, 2022 (PDF )
Suzuki H, Ohishi T, Asano T, Tanaka T, Saito M, Mizuno T, Yoshikawa T, Kawada M, Kaneko MK, Kato Y.
Oncol. Rep., ,48(3), 154, https://doi.org/10.3892/or.2022.8366, 2022 (PDF )
Saito M, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 157–162, https://doi.org/10.1089/mab.2022.0013, 2022 (PDF )
8. Tanaka T, Li G, Asano T, Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 150–156, https://doi.org/10.1089/mab.2022.0012, 2022 (PDF )
7. Goto N, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 163–169, https://doi.org/10.1089/mab.2022.0014, 2022 (PDF )
6. Kitamura K, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 133–141, https://doi.org/10.1089/mab.2022.0010, 2022 (PDF )
5. Li G, Suzuki H, Asano T, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
Antibodies , 11(2), 41; https://doi.org/10.3390/antib11020041, 2022 (PDF ) (Preprint )
4. Goto N, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Int. J. Mol. Sci., 23(10), 5535; https://doi.org/10.3390/ijms23105535, 2022 (PDF ) (Preprint )
2021
Asano T, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , DOI: 10.1089/mab.2021.0030, 2021 (ref )2020
Kaneko MK, Ohishi T, Kawada M, Kato Y.
Biochem Biophys Rep., 24, 100826, https://doi.org/10.1016/j.bbrep.2020.100826, 2020 (PDF )Kaneko MK, Ohishi T, Nakamura T, Inoue H, Takei J, Sano M, Asano T, Sayama Y, Hosono H, Suzuki H, Kawada M, Kato Y.* Monoclon. Antib. Immunodiagn. Immunother. , 39(5), 167–174, https://doi.org/10.1089/mab.2020.0019, 2020 (PDF )
動画(YouTube)
総説(pdf)
AMED project