
Histol Histopathol. 2013 Mar;28(3):293-9.
Role of podoplanin expression in squamous cell carcinoma of upper aerodigestive tract.
Chuang WY, Chang YS, Yeh CJ, Wu YC, Hsueh C.
Department of Pathology, Chang Gung Memorial Hospital, Linko, Chang Gung University College of Medicine, Taoyuan, Taiwan.
Abstract
Podoplanin, a type-1 transmembrane glycoprotein, was originally named due to its expression in renal podocytes of rats. It was subsequently detected in a variety of normal human tissues, including lymphatic endothelium. Although podoplanin has been identified as the endogenous ligand of C-type lectin-like receptor 2 (CLEC-2) on platelets, its physiological functions and pathways remain largely unknown. A role in lymphangiogenesis has been suggested, since podoplanin-deficient mice were found to die at birth with a phenotype of dilated, malfunctioning lymphatic vessels and lymphedema. Podoplanin is invariably expressed in some tumors, such as lymphangioma, seminoma and follicular dendritic cell tumor, but tumor cell expression of podoplanin is highly variable in squamous cell carcinoma (SCC). It has been found that high podoplanin expression is associated with lymph node metastasis and poor prognosis in SCC of the upper aerodigestive tract. Now there is growing evidence that podoplanin is also involved in carcinogenesis, cell motility, tumor invasiveness, platelet aggregation and hematogenous metastasis. Additionally, animal studies confirmed some in vivo effects of podoplanin-overexpressing tumors, including formation of more tumor lymphatic vessels, larger lymph node metastases, more platelet aggregation, and more pulmonary metastases. Several recently developed anti-podoplanin antibodies, such as NZ-1, P2-0 and hP2-0, have been shown to attenuate podoplanin-induced platelet aggregation and prevent experimental hematogenous metastasis in nude mice. These antibodies may be applied in preclinical and clinical studies to evaluate the possibility of podoplanin-targeted therapy.
J Biol Chem. 2013 Jan 22. [Epub ahead of print]
Fucoidan is a novel platelet agonist for the CLEC-2 receptor.
Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, Naik U, Watson SP, Kunapuli SP.
Temple University, United States;
Abstract
Fucoidan, a sulphated polysaccharide from fucus vesiculosus decreases bleeding time and clotting time in hemophilia, possibly through inhibition of tissue factor pathway inhibitor (TFPI). However, its effect on platelets and the receptor by which fucoidan induces cellular processes has not been elucidated. In this study, we demonstrate that fucoidan induces platelet activation in a concentration-dependent manner. Fucoidan-induced platelet activation was completely abolished by the pan-Src family kinase (SFK) inhibitor, PP2, or when Syk is inhibited. PP2 also abolished phosphorylations of Syk and PLC-gamma 2. Furthermore, fucoidan-induced platelet activation had a lag phase, which is reminiscent of platelet activation by collagen and by CLEC-2 receptor agonists. Platelet activation by fucoidan however was only slightly inhibited in FcRgamma-chain null mice indicating that fucoidan is not acting primarily through GPVI receptor. On the other hand, fucoidan-induced platelet activation was inhibited in platelet specific CLEC-2 knockout murine platelets revealing CLEC-2 as a physiological target of fucoidan. Thus, our data shows fucoidan as a novel CLEC-2 receptor agonist that activates platelets through an SFK-dependent signaling pathway. Furthermore, the efficacy of fucoidan in hemophilia raises the possibility that decreased bleeding times could be achieved through activation of platelets.
Oncol Rep. 2013 Jan 4. doi: 10.3892/or.2013.2225. [Epub ahead of print]
Podoplanin overexpression in human mesothelioma cell lines enhances the tumorigenic phenotype.
Yamaki E, Yajima T, Kosaka T, Mogi A, Tanaka S, Kuwano H.
Department of General Surgical Science, Gunma University Graduate School of Medicine, Gunma, Japan.
Abstract
Podoplanin, a small type I integral membrane mucin-type sialoglycoprotein, serves as a useful marker for diagnosing malignant pleural mesothelioma (MPM); however, the physiological function of podoplanin in mesothelioma cells is not known. To elucidate the role of podoplanin in the pathogenesis of MPM, we generated two mesothelioma cell lines (PODO1 and PODO2) that stably express high levels of podoplanin. Although PODO1 cells proliferated to the same extent in culture or in nude mice, the survival rate of the mice was significantly reduced compared with that of the controls. We demonstrated that PODO1 and PODO2 cells had increased invasive ability in in vitro assays and induced upregulation of matrix metalloproteinase-1. PODO1 and PODO2 cultures could not be induced to undergo apoptosis when starved or treated with cis-diamminedichloroplatinum(II) (CDDP) compared with the controls. Moreover, silencing of podoplanin expression using RNA interference restored the ability of CDDP to induce apoptosis. Consistent with their growth properties, we detected constitutive activation of extracellular signal-regulated kinase in PODO1 and PODO2 cultures. These findings suggest that constitutive expression of podoplanin contributes to the invasive growth properties of mesothelioma cells and their resistance to apoptosis. Moreover, our data suggest that podoplanin or components of its signaling pathway, or both, may serve as important targets for developing novel treatments for MPM.
Mol Immunol. 2012 Dec 31;54(2):199-207. doi: 10.1016/j.molimm.2012.11.013. [Epub ahead of print]
Podoplanin is an inflammatory protein upregulated in Th17 cells in SKG arthritic joints.
Miyamoto Y, Uga H, Tanaka S, Kadowaki M, Ikeda M, Saegusa J, Morinobu A, Kumagai S, Kurata H.
Sysmex Corporation, Japan; Department of Rheumatology and Clinical Immunology, Kobe University Graduate School of Medicine, Japan.
Abstract
Interleukin 17-producing helper T (Th17) cells play pathogenic roles in chronic inflammatory and autoimmune diseases, including arthritis, colitis and multiple sclerosis. Th17 cells selectively express the transcription factor RORγt, as well as the cytokine receptors IL-23R and CCR6. Identification of novel Th17 cell-specific molecules may have potential value as diagnostic markers in the above-mentioned inflammatory diseases. To that aim, we carried out a comparative microarray analysis on in vitro differentiated Th1, Th2, Treg and Th17 cells from naive CD4(+) cells of BALB/c mice. Among a total of one hundred and twenty Th17 cell-specific molecules, twenty-nine were novel cell-surface molecules. Then we revealed that thirteen of them were up-regulated in vivo in inflamed tissues from experimental autoimmune diseases, including spontaneous SKG arthritis, inflammatory bowel disease (IBD) and experimental autoimmune encephalomyelitis (EAE). Next, we analyzed the expression of four membranous molecules, and revealed that podoplanin was expressed highly in the in vitro differentiated Th17 cells. Moreover, at the inflamed synovium of the arthritic SKG mice, most of the accumulating Th17 cells were podoplanin-positive. These results indicate that podoplanin would be a useful Th17 cell marker for diagnosing pathological conditions of autoimmune diseases, including rheumatoid arthritis.
*我々の論文(Mishima K, Kato Y et al., ANP 2006)がNature Medに引用されました。
Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy.
Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H.
Nat Med. 2012 Dec;18(12):1835-40. doi: 10.1038/nm.2994. Epub 2012 Nov 11. PMID: 23142818
J Biol Chem. 2013 Mar 25. [Epub ahead of print]
Serines in the Intracellular Tail of Podoplanin (PDPN) Regulate Cell Motility.
Krishnan H, Ochoa-Alvarez JA, Shen Y, Nevel E, Lakshminarayanan M, Williams MC, Ramirez MI, Miller WT, Goldberg GS.
UMDNJ, United States;
Abstract
Podoplanin (PDPN) is a transmembrane receptor that affects the activities of RHO, ezrin, and other proteins to promote tumor cell motility, invasion, and metastasis. PDPN is found in many types of cancer and may serve as a tumor biomarker and chemotherapeutic target. The intracellular region of PDPN contains only two serines, and these are conserved in mammals including mice and humans. We generated cells from the embryos of homozygous null PDPN knockout mice to investigate the relevance of these serines to cell growth and migration on a clear (PDPN free) background. We report here that one or both of these serines can be phosphorylated by PKA (protein kinase A). We also report that conversion of these serines to nonphosphorylatable alanine residues enhances cell migration, while their conversion to phosphomimetic aspartate residues decreases cell migration. These results indicate that PKA can phosphorylate PDPN to decrease cell migration. In addition, we report that PDPN expression in fibroblasts causes them to facilitate the motility and viability of neighboring melanoma cells in coculture. These findings shed new light on how PDPN promotes cell motility, its role in tumorigenesis, and its utility as a functionally relevant biomarker and chemotherapeutic target.
Neuro Oncol. 2013 Mar 13. [Epub ahead of print]
STAT3 silencing inhibits glioma single cell infiltration and tumor growth.
Priester M, Copanaki E, Vafaizadeh V, Hensel S, Bernreuther C, Glatzel M, Seifert V, Groner B, Kogel D, Weissenberger J.
Experimental Neurosurgery, Goethe University Hospital, Neuroscience Center, Frankfurt, Germany (M.P., S.H., D.K., J.W.); Institute of Clinical Neuroanatomy, Goethe University, Neuroscience Center, Frankfurt, Germany (E.C.); Georg-Speyer-Haus, Institute for Biomedical Research, Frankfurt, Germany (V.V., B.G.); Institute of Neuropathology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (C.B., M.G.); Department of Neurosurgery, Center of Neurology and Neurosurgery, Goethe University Hospital, Frankfurt, Germany (V.S.).
Abstract
BackgroundDiffuse infiltration remains the fulcrum of glioblastoma's incurability, leading inevitably to recurrence. Therefore, uncovering the pathological mechanism is imperative. Because signal transducer and activator of transcription 3 (STAT3) correlates with glioma malignancy and predicts poor clinical outcome, we determined its role in glioma single cell infiltration and tumor growth.MethodsSTAT3 was silenced in Tu-2449 glioma cells via lentiviral gene transfer. Target gene expression was measured by real-time reverse transcription PCR, Western blotting, and immunohistochemistry. Microvilli were visualized by staining with wheat germ agglutinin. Migration and invasion were measured by Scratch and Matrigel chamber assays. Diffuse infiltration was studied in 350-μm-thick organotypic tissue cultures over 14 days using cells tagged with enhanced green fluorescent protein and live confocal laser scanning microscopy. Survival of tumor-bearing syngeneic, immunocompetent B6C3F1 mice was analyzed by Kaplan-Meier plots.ResultsSTAT3 silencing reduced cell migration and invasion in vitro and stopped single cell infiltration ex vivo, while STAT3-expressing cells disseminated through the neuropil at ?100 μm/day. STAT3 silencing reduced transcription of several tumor progression genes. Mice with intracranial STAT3 knockdown tumors had a significant (P< .0007) survival advantage over controls, yielding 27% long-term survival. STAT3 knockdown reduced podoplanin expression 50-fold and inhibited concurrent microvilli formation. STAT3 knockdown tumors exhibited a weaker podoplanin immunoreactivity compared with controls. Podoplanin staining was diffuse, preferentially at tumor margins, and absent in normal brain.ConclusionsOur results show compelling evidence that STAT3 is a key driver of diffuse infiltration and glioma growth and might therefore represent a promising target for an anti-invasive therapy.
Biochem Biophys Res Commun. 2013 Mar 26. pii: S0006-291X(13)00495-6. doi: 10.1016/j.bbrc.2013.03.057. [Epub ahead of print]
Extracellular heat shock protein A9 is a novel interaction partner of podoplanin in oral squamous cell carcinoma cells.
Tsuneki M, Maruyama S, Yamazaki M, Xu B, Essa A, Abe T, Babkair H, Cheng J, Yamamoto T, Saku T.
Division of Oral Pathology, Department of Tissue Regeneration and Reconstruction, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan; Fellow, the Japan Society for the Promotion of Science, Japan.
Abstract
In previous studies, we have shown several lines of evidence that podoplanin (PDPN) plays an important role in cell adhesion via its association with extracellular components in neoplastic conditions, though there has been no trial for searching PDPN-interaction molecules in the extracellular milieu. To screen for those molecules, we performed proteomics-based analysis using liquid chromatography-tandem mass spectrometry followed by co-immunoprecipitation for PDPN in ZK-1, an oral squamous cell carcinoma (SCC) cell system whose cell membrane molecules were cross-linked each other in their extracellular compartments, and we identified heat shock protein (HSP) A9 as one of the extracellular PDPN bound molecules. Effects of transient PDPN knockdown by siRNA in ZK-1 were also comparatively examined for cellular behaviors in terms of HSPA9 expression and secretion. Finally, HSPA9 expression modes were immunohistochemically visualized in oral SCC tissue specimens. HSPA9 was secreted from ZK-1 cells, and the expression and secretion levels of HSPA9 gene and protein were well coordinated with those of PDPN. Immunohistochemically, HSPA9 and PDPN were co-localized in ZK-1 cells and oral SCC foci, especially in the peripheral zone. In conclusion, the results indicate that HSPA9 secreted by oral SCC cells interacts with PDPN on their cell surface in an autocrine manner and regulates their growth and invasiveness.
Lab Invest. 2013 Jul 1. doi: 10.1038/labinvest.2013.86. [Epub ahead of print]
Podoplanin-mediated cell adhesion through extracellular matrix in oral squamous cell carcinoma.
Tsuneki M, Yamazaki M, Maruyama S, Cheng J, Saku T.
Division of Oral Pathology, Department of Tissue Regeneration and Reconstruction, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan.
Abstract
Podoplanin (PDPN), one of the representative mucin-like type-I transmembrane glycoproteins specific to lymphatic endothelial cells, is expressed in various cancers including squamous cell carcinoma (SCC). On the basis of our previous studies, we have developed the hypothesis that PDPN functions in association with the extracellular matrix (ECM) from the cell surface side. The aim of this study was to elucidate the molecular role of PDPN in terms of cell adhesion, proliferation, and migration in oral SCC cells. Forty-four surgical specimens of oral SCC were used for immunohistochemistry for PDPN, and the expression profiles were correlated with their clinicopathological properties. Using ZK-1, a human oral SCC cell system, and five other cell systems, we examined PDPN expression levels by immunofluorescence, western blotting, and real-time PCR. The effects of transient PDPN knockdown by siRNA in ZK-1 were determined for cellular functions in terms of cell proliferation, adhesion, migration, and invasion in association with CD44 and hyaluronan. Cases without PDPN-positive cells were histopathologically classified as less-differentiated SCC, and SCC cells without PDPN more frequently invaded lymphatics. Adhesive properties of ZK-1 were significantly inhibited by siRNA, and PDPN was shown to collaborate with CD44 in cell adhesion to tether SCC cells with hyaluronan-rich ECM of the narrow intercellular space as well as with the stromal ECM. There was no siRNA effect in migration. We have demonstrated the primary function of PDPN in cell adhesion to ECM, which is to secondarily promote oral SCC cell proliferation
Int J Surg Pathol. 2013 Jul 1. [Epub ahead of print]
Podoplanin and Clusterin Are Reliable Markers of Nonneoplastic Synovium at Various Sites.
Talmon GA, Wake L, Muirhead D.
Abstract
Introduction. Clusterin (CLU) has been noted to mark synovium adjacent to tenosynovial tumors, and studies suggest that podoplanin (PP) is upregulated in inflammatory arthritis. Characterization of synovial staining with CLU and PP in various nonneoplastic disease states has not been described. Methods. A microarray was created from paraffin-embedded human synovium, including 19 normal/noninflammatory (10 weight-bearing joints, 8 non-weight-bearing joints), 9 rheumatoid arthritis, 10 synovial cysts, and 3 osteoarthritis and stained with PP (D2-40) and CLU. Staining intensity was graded semiquantitatively (0-3+). Results. PP and CLU stained synovium in 88% and 95% cases, respectively. PP and CLU showed moderate to strong (3+) staining in 26% and 19% of noninflammatory and 44% and 0% of inflammatory synovia, respectively (P < .01). Conclusions. PP and CLU are reliable markers of human synovium and can confirm its presence in limited specimens. Although CLU was more sensitive, PP may be more useful in the setting of chronic inflammation.
In Vivo. 2013 Jul-Aug;27(4):551-4.
Enhanced podoplanin expression in chronic maxillary sinusitis.
Zustin J, Scheuer HA, Knecht R, Friedrich RE.
Institute of Pathology, University Medical Centre Hamburg Eppendorf, Martinistr.52, 20246 Germany. j.zustin@uke.uni-hamburg.de.
Abstract
Podoplanin expression has been reported in oral squamous epithelium, myoepithelia of the salivary glands, and odontogenic lesions, and has been linked with inflammatory and neoplastic conditions. We hypothesized that inflamed respiratory mucosa of the maxillary sinus also express podoplanin, especially in cases with odontogenic sinusitis. We retrospectively investigated podoplanin expression in biopsies from maxillary sinus with inflammatory changes. Cases with chronic rhinosinusitis with polyp formation (n=5), chronic rhinosinusitis without polyps (n=5), chronic rhinosinusitis with eosinophilia (n=5), and odontogenic chronic rhinosinusitis (n=5) were investigated immunohistochemically using an established antibody for podoplanin (D2-40). Respiratory epithelium in chronic maxillary sinusitis with polyp formation did not exhibit enhanced podoplanin expression. However, D2-40 positivity was detected in the basal cells in all cases with chronic sinusitis associated with inflammatory infiltrations as well as in the parabasal epithelial layer in chronic sinusitis without polyp formation. We observed podoplanin expression in non-neoplastic maxillary sinus epithelium exhibiting inflammatory changes. We suggest that podoplanin is involved in the pathogenesis of chronic rhinosinusitis, particularly in the intraepithelial migration of inflammatory infiltrates.
Immunol Lett. 2013 Jul 30. pii: S0165-2478(13)00099-0. doi: 10.1016/j.imlet.2013.07.007. [Epub ahead of print]
Thymic medullar conduits-associated podoplanin promotes natural regulatory T cells.
Fuertbauer E, Zaujec J, Uhrin P, Raab I, Weber M, Schachner H, Bauer M, Schutz GJ, Binder BR, Sixt M, Kerjaschki D, Stockinger H.
Molecular Immunology Unit, Institute for Hygiene and Applied Immunology, Centre for Pathophysiology, Infectiology and Immunology, Medical University of Vienna, Lazarettgasse 19, 1090 Vienna, Austria.
Abstract
Podoplanin, a mucin-like plasma membrane protein, is expressed by lymphatic endothelial cells and responsible for separation of blood and lymphatic circulation through activation of platelets. Here we show that podoplanin is also expressed by thymic fibroblastic reticular cells (tFRC), a novel thymic medulla stroma cell type associated with thymic conduits, and involved in development of natural regulatory T cells (nTreg). Young mice deficient in podoplanin lack nTreg owing to retardation of CD4+CD25+ thymocytes in the cortex and missing differentiation of Foxp3+ thymocytes in the medulla. This might be due to CCL21 that delocalizes upon deletion of the CCL21-binding podoplanin from medullar tFRC to cortex areas. The animals do not remain devoid of nTreg but generate them delayed within the first month resulting in Th2-biased hypergammaglobulinemia but not in the death-causing autoimmune phenotype of Foxp3-deficient Scurfy mice.
*ポドプラニン/CLEC-2関連の論文がNatureに出ました。(Nature)
ポドプラニンの生理的な機能を証明した論文としては、初めての報告かもしれません。これまでは、ポドプラニンがCLEC-2と結合して血小板凝集を引き起こすことがリンパ管の発生に関わるという話がありましたが、今回はそれを超えるインパクトです。
ポドプラニン/CLEC-2のinteractionを初めて報告したのは、山梨大学の井上先生/尾崎先生と我々のグループの共同研究ですが、今回の論文で引用されているのは、後発のヨーロッパの仕事なのが少々残念です。日本人の論文が一切引用されていません。まだまだ我々の力不足を感じますが、他人の仕事を羨ましく思っている暇はありません。我々独自の仕事を次々に世の中に出すことが大切だと思います。
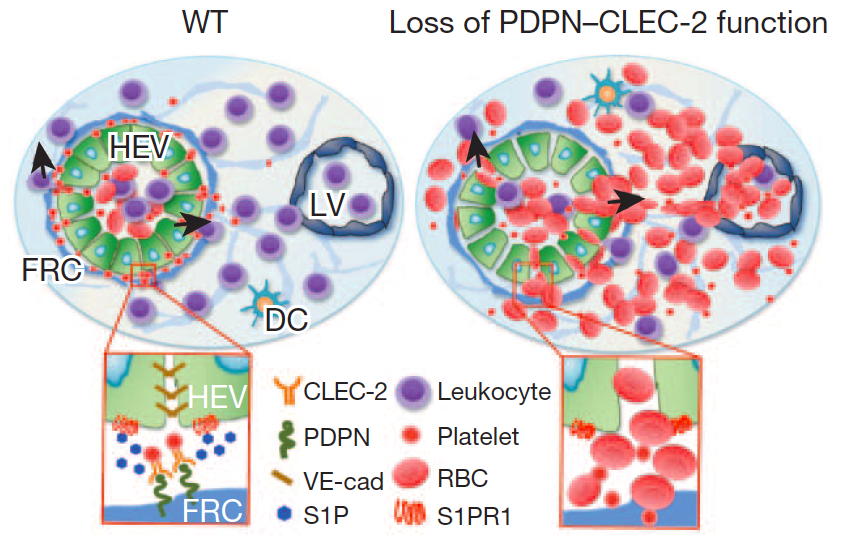
*高内皮細静脈からのリンパ球遊出機構に血小板が関与を発見
免疫系の細胞であるリンパ球は、血管(高内皮細静脈:HEV)壁を通過してリンパ節に移動するが、出血を伴うことなくリンパ球が大量に血管外に出る仕組みは明らかではなかった。米Oklahoma医学研究財団(OMRF)のLijun Xia博士らは、この過程にポドプラニンと血小板CLEC-2などが関与することを明らかにした。詳細はNature誌電子版に、2013年9月1日に報告された。 これまで、血小板の役割は出血時に凝集して血栓を形成、出血を止めることだと考えられてきたが、今回新たに、HEVの完全性を損なうことなくリンパ球の遊出を促すことによって、免疫応答にも関係していることが明らかになった。 著者らは、免疫反応が起きるとリンパ球のリンパ節への移動が増えるが、HEVの組織が正常な状態を維持したまま、これを可能にする方法を明らかにしようと考えた。 研究者たちは最初に、膜貫通型の糖たんぱく質ポドプラニン(PDPN、gp38、T1αとも呼ばれる)がHEVの完全性の維持に役割を果たしていることを明らかにした。PDPN遺伝子を欠くマウスではHEVの構造が変化しており、粘膜リンパ節内での突発的な出血が発生、免疫刺激後には、流入領域末梢リンパ節での出血が見られた。リンパ球のホーミング(再循環)を阻止すると出血は回避されたことから、PDPNはリンパ球のトラフィッキングの間のHEVの完全性の維持に必要と考えられた。 次に、PDPNはHEVを取り巻く線維芽球性網状細胞の表面に発現されていることが明らかになり、PDPNが血小板に存在するC型レクチン様受容体2(CLEC-2。CLEC1Bとも呼ばれる)の活性化リガンドとして機能することも判明。さらに、線維芽球性網状細胞、PDPN、血小板CLEC-2の3つが、血管の完全性に欠かせない血管内皮(VE)カドヘリン(CDH5とも呼ばれる)のHEVにおける発現に必要であることが示された。また、ex vivoで、CLEC-2を活性化すると、血小板からのスフィンゴシン-1-リン酸の放出が誘導され、これによりHEV表面のVEカドヘリンの発現が上昇。さらに、スフィンゴシン-1-リン酸欠損マウスを免疫刺激したところ、流入領域末梢リンパ節でHEVの完全性が損なわれ、PDPNとCLEC-2の両方が欠損しているマウスと同様の特徴を示した。 これらのデータは、PDPNと血小板CLEC-2に仲介される血小板活性化後に局所で生じるスフィンゴシン-1-リン酸の放出が、免疫反応中のHEVの完全性に必須であることを示した。 得られた知見は、血小板が止血のみならず免疫反応にも関わることを示し、血小板を利用した重症感染症治療法の開発などが可能であることを示唆した。
PLoS One. 2013 Aug 21;8(8):e73609. doi: 10.1371/journal.pone.0073609.(PLoS One)
Platelets Promote Tumor Growth and Metastasis via Direct Interaction between Aggrus/Podoplanin and CLEC-2.
Takagi S, Sato S, Oh-Hara T, Takami M, Koike S, Mishima Y, Hatake K, Fujita N.
Division of Experimental Chemotherapy, Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, Tokyo, Japan.
Abstract The platelet aggregation-inducing factor Aggrus, also known as podoplanin, is frequently upregulated in several types of tumors and enhances hematogenous metastasis by interacting with and activating the platelet receptor CLEC-2. Thus, Aggrus-CLEC-2 binding could be a therapeutic molecular mechanism for cancer therapy. We generated a new anti-human Aggrus monoclonal antibody, MS-1, that suppressed Aggrus-CLEC-2 binding, Aggrus-induced platelet aggregation, and Aggrus-mediated tumor metastasis. Interestingly, the MS-1 monoclonal antibody attenuated the growth of Aggrus-positive tumors in vivo. Moreover, the humanized chimeric MS-1 antibody, ChMS-1, also exhibited strong antitumor activity against Aggrus-positive lung squamous cell carcinoma xenografted into NOD-SCID mice compromising antibody-dependent cellular cytotoxic and complement-dependent cytotoxic activities. Because Aggrus knockdown suppressed platelet-induced proliferation in vitro and tumor growth of the lung squamous cell carcinoma in vivo, Aggrus may be involved in not only tumor metastasis but also tumor growth by promoting platelet-tumor interaction, platelet activation, and secretion of platelet-derived factors in vivo. Our results indicate that molecular target drugs inhibiting specific platelet-tumor interactions can be developed as antitumor drugs that suppress both metastasis and proliferation of tumors such as lung squamous cell carcinoma.
*久しぶりに、正しく(?)我々の論文が引用されていました。
PLoS One. 2013 Oct 9;8(10):e77259.
Podoplanin Immunopositive Lymphatic Vessels at the Implant Interface in a Rat Model of Osteoporotic Fractures.
Lips KS, Kauschke V, Hartmann S, Thormann U, Ray S, Kampschulte M, Langheinrich A, Schumacher M, Gelinsky M, Heinemann S, Hanke T, Kautz AR, Schnabelrauch M, Schnettler R, Heiss C, Alt V, Kilian O.
Laboratory of Experimental Trauma Surgery, Justus-Liebig University, Giesen, Germany.
Abstract
Insertion of bone substitution materials accelerates healing of osteoporotic fractures. Biodegradable materials are preferred for application in osteoporotic patients to avoid a second surgery for implant replacement. Degraded implant fragments are often absorbed by macrophages that are removed from the fracture side via passage through veins or lymphatic vessels. We investigated if lymphatic vessels occur in osteoporotic bone defects and whether they are regulated by the use of different materials. To address this issue osteoporosis was induced in rats using the classical method of bilateral ovariectomy and additional calcium and vitamin deficient diet. In addition, wedge-shaped defects of 3, 4, or 5 mm were generated in the distal metaphyseal area of femur via osteotomy. The 4 mm defects were subsequently used for implantation studies where bone substitution materials of calcium phosphate cement, composites of collagen and silica, and iron foams with interconnecting pores were inserted. Different materials were partly additionally functionalized by strontium or bisphosphonate whose positive effects in osteoporosis treatment are well known. The lymphatic vessels were identified by immunohistochemistry using an antibody against podoplanin. Podoplanin immunopositive lymphatic vessels were detected in the granulation tissue filling the fracture gap, surrounding the implant and growing into the iron foam through its interconnected pores. Significant more lymphatic capillaries were counted at the implant interface of composite, strontium and bisphosphonate functionalized iron foam. A significant increase was also observed in the number of lymphatics situated in the pores of strontium coated iron foam. In conclusion, our results indicate the occurrence of lymphatic vessels in osteoporotic bone. Our results show that lymphatic vessels are localized at the implant interface and in the fracture gap where they might be involved in the removal of lymphocytes, macrophages, debris and the implants degradation products. Therefore the lymphatic vessels are involved in implant integration and fracture healing.
*相変わらず、D2-40という抗体の名前を分子名として使っている論文が掲載されています。reviewerが直すべきでしょう。
Hum Pathol. 2013 Oct 11. pii: S0046-8177(13)00311-0. doi: 10.1016/j.humpath.2013.07.022. [Epub ahead of print]
Association of D2-40 and MMP-1 expression with cyst formation in lung metastatic lesions of cutaneous angiosarcoma on the scalp: immunohistochemical analysis of 23 autopsy cases.
Masuzawa M, Mikami T, Numata Y, Tokuyama W, Masuzawa M, Murakumo Y, Okayasu I, Katsuoka K.
Department of Dermatology, Kitasato University School of Medicine, Sagamihara, Kanagawa 252-0374, Japan. Electronic address: masuderm@med.kitasato-u.ac.jp.
Abstract
Cutaneous angiosarcoma of the scalp can rapidly develop into pulmonary metastasis. The pulmonary metastatic lesions display a unique appearance, so-called thin-walled cysts, which cause a fatal relapsed pneumothorax by rupturing. We analyzed 23 autopsy cases of angiosarcoma with pulmonary metastasis to elucidate the mechanism of the thin-walled cyst development. Of the 23 cases of cutaneous angiosarcoma of the scalp with pulmonary metastasis, radiological examination revealed pulmonary metastatic lesions as thin-walled cysts (39%), nodules (39%), mixed cysts and nodules (13%), and ground-glass opacity (9%). All the cases but one with cystic metastases were complicated by pneumothorax. The cystic lesions were accompanied by podoplanin (D2-40)-positive tumor cells in the luminal surface of the cysts. In both primary cutaneous lesions and pulmonary metastatic lesions, the D2-40 expression was positive for angiosarcoma cells in 100% and 92% of the cases, respectively. While the estrogen-regulated gene (ERG) expression was also positive for most of the primary and metastatic pulmonary angiosarcomas, D2-40 was a more useful marker to differentiate tumor cells from the background than was the ERG expression of the vascular endothelium. Matrix metalloproteinase-1 (MMP-1) expression was also predominant in primary lesions (95%) and pulmonary metastatic lesions (82.6%). Proteinases, like MMP-1, might be associated with a developing thin-walled cyst, although there were no differences in the MMP-1 expression in either the cystic or nodular metastasis. Two extremely aggressive cases showed cystic metastasis with central necrosis that was not observed in other cases. These results suggest a pathogenesis of thin-walled cysts in some progressive cases.
Cancer Sci. 2013 Sep 16. doi: 10.1111/cas.12286. [Epub ahead of print]
Podoplanin is Expressed at the Invasive Front of Esophageal Squamous Cell Carcinomas and is Involved in Collective Cell Invasion.
Nakashima Y, Yoshinaga K, Kitao H, Ando K, Kimura Y, Saeki H, Oki E, Morita M, Kakeji Y, Hirahashi M, Oda Y, Maehara Y.
Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University.
Abstract
The expression of podoplanin is reportedly involved in collective cell invasion, which is independent from the epithelial-mesenchymal transition (EMT). We focused on the expression of podoplanin in esophageal squamous cell carcinomas (ESCC) and investigated the correlation of podoplanin and EMT-related markers, and evaluated its prognostic significance. Five ESCC cell lines were subjected to Western blot analysis for podoplanin and EMT markers. The effects of podoplanin on EMT and carcinoma invasion were evaluated with wound healing assays, invasion assays, and three-dimensional (3D) culture. Transfection of ectopic podoplanin into a podoplanin-negative ESCC cell line (TE-15) induced cell migration and invasive activity (p < 0.001 and p < 0.05, respectively) without downregulation of E-cadherin. In contrast, transfection of si-podoplanin RNA into a podoplanin-positive ESCC cell line (TE-13) reduced cell migration and invasive activity (p < 0.05). We reviewed 101 patients who had undergone esophagectomy for ESCC. Podoplanin expression was observed in 58 patients (57.4%), and positive expression was positively correlated with expression of E-cadherin (p < 0.01), deeper wall invasion (p < 0.01), venous invasion (p < 0.05), and poorer prognosis (p < 0.01). Multivariate Cox analysis revealed that expression of podoplanin was a significant and independent unfavorable predictor of survival (p < 0.05). These data suggest that podoplanin is significantly associated with and likely contributes to ESCC invasion in the absence of EMT.
Int J Biochem Cell Biol. 2013 Nov 22. pii: S1357-2725(13)00354-3. doi: 10.1016/j.biocel.2013.11.016. [Epub ahead of print]
Podoplanin is a substrate of presenilin-1/γ-secretase.
Yurrita MM, Fernandez-Munoz B, Del Castillo G, Martin-Villar E, Renart J, Quintanilla M.
Instituto de Investigaciones Biomedicas Alberto Sols, Consejo Superior de Investigaciones Cientificas (CSIC)-Universidad Autonoma de Madrid (UAM), 28029-Madrid, Spain.
Abstract
Podoplanin (PDPN) is a mucin-like transmembrane glycoprotein that plays an important role in development and cancer. Here, we provide evidence that the intracellular domain (ICD) of podoplanin is released into the cytosol following a sequential proteolytic processing by a metalloprotease and γ-secretase. Western blotting and cell fractionation studies revealed that HEK293T and MDCK cells transfected with an eGFP-tagged podoplanin construct (PDPNeGFP, 50-63kDa) constitutively express two C-terminal fragments (CTFs): a ?33kDa membrane-bound PCTF33, and a ?29kDa cytosolic podoplanin ICD (PICD). While pharmacological inhibition of metalloproteases reduced the expression of PCTF33, treatment of cells with γ-secretase inhibitors resulted in enhanced PCTF33 levels. PCTF33 processing by γ-secretase depends on presenilin-1 (PS1) function: cells expressing a dominant negative form of PS1 (PS1 D385N), and mouse embryonic fibroblasts (MEFs) genetically deficient in PS1, but not in PS2, show higher levels of PCTF33 expression with respect to wild-type MEFs. Furthermore, transfection of PS1 deficient MEFs with wild-type PS1 (PS1wt) decreased PCTF33 levels. N-terminal amino acid sequencing of the affinity purified PICD revealed that the γ-secretase cleavage site was located between valines 150 and 151, but these residues are not critical for proteolysis. We found that podoplanin CTFs are also generated in cells expressing podoplanin mutants harboring heterologous transmembrane regions. Taken together, these results indicate that podoplanin is a novel substrate for PS1/γ-secretase.
*Mol. Cancerは、IFが5を超える雑誌のようです。我々の論文が2報引用されています。(ただ、またしても、適切な引用ではありませんが。。。)論文では、eBioscienceから販売されているNZ-1.3(加藤が作製した抗体)が使われています。NZ-1.3はNZ-1と同等の活性を持つ良い抗体です。
Mol Cancer. 2013 Dec 20;12(1):168. [Epub ahead of print]
Podoplanin expression in cancer-associated fibroblasts enhances tumor progression of invasive ductal carcinoma of the pancreas.
Shindo K, Aishima S, Ohuchida K, Fujiwara K, Fujino M, Mizuuchi Y, Hattori M, Mizumoto K, Tanaka M, Oda Y.
Abstract
BACKGROUND:
Interactions between cancer cells and surrounding cancer-associated fibroblasts (CAFs) play an important role in cancer progression. Invasive ductal carcinoma (IDC) of the pancreas is characterized by abundant fibrous connective tissue called desmoplasia. Podoplanin (PDPN) is a lymphatic vessel marker (D2-40), and expression of PDPN by stromal CAFs has been reported to be a prognostic indicator in various types of cancer.
METHODS:
Expression of PDPN in pancreatic IDCs was assessed by immunohistochemical examination in 105 patients who underwent pancreatic resection. Primary CAFs were established from pancreatic cancer tissue obtained by surgery. Quantitative reverse transcription-polymerase chain reaction and flow cytometric analysis were performed to investigate PDPN expression in CAFs. We sorted CAFs according to PDPN expression, and analyzed the functional differences between PDPN+ CAFs and PDPN- CAFs using indirect co-culture with pancreatic cancer cell lines. We also investigated the culture conditions to regulate PDPN expression in CAFs.
RESULTS:
PDPN expression in stromal fibroblasts was associated with lymphatic vessel invasion (P = 0.0461), vascular invasion (P = 0.0101), tumor size >=3 cm (P = 0.0038), histological grade (P = 0.0344), Union for International Cancer Control classification T stage (P = 0.029), and shorter survival time (P < 0.0001). Primary CAFs showed heterogeneous PDPN expression in vitro. Moreover, migration and invasion of pancreatic cancer cell lines (PANC-1 and SUIT-2) were associated with PDPN expression in CAFs (P < 0.01) and expression of CD10, matrix metalloproteinase (MMP) 2, and MMP3. In cultured CAFs, PDPN positivity changed over time under several conditions including co-culture with cancer cells, different culture media, and addition of growth factor.
CONCLUSIONS:
PDPN-expressing CAFs enhance the progression of pancreatic IDC, and a high ratio of PDPN-expressing CAFs is an independent predictor of poor outcome. Understanding the regulation of the tumor microenvironment is an important step towards developing new therapeutic strategies.