
名古屋大学脳神経外科、慶応大学先端研との共同研究の論文がTumor Biologyにアクセプトされました。
以前の論文で、<mutant IDH1/2の下流にAktのシグナルが動き、さらにポドプラニンの発現が動く>、という論文があるらしく、その文献の流れにそった形で検証が行われている。我々の論文2報(Mishima_ANP_2006, Motomura_CAS_2012)が引用され、ポドプラニンのWBにおける検出に、NZ-1.2が使用されている。ただ、そのWBのバンドを見ると、本当にポドプラニンのバンドかどうか怪しい。最近、ポドプラニンのバンドというのが、とてもシャープなバンドの論文が多く、しかも、分子量が示されていないので、ポドプラニンのバンドでない可能性がある。今回の論文では、免疫染色ではD2-40を使っているのに、WBではNZ-1.2を使っていることから、D2-40ではバンドが検出されなかったものと考えられる。NZ-1.2の方がD2-40よりも感度が高いのは確かだが、D2-40で検出されなかったため、NZ-1.2をWBのみで使い、出たバンドの一部を切り取った可能性がある。いずれにしても、不可解なデータが多い。Cancerという有名雑誌であるが…。
Mutant IDH1 inhibits PI3K/Akt signaling in human glioma.
Birner P, Pusch S, Christov C, Mihaylova S, Toumangelova-Uzeir K, Natchev S, Schoppmann SF, Tchorbanov A, Streubel B, Tuettenberg J, Guentchev M.
Cancer. 2014 Apr 25. doi: 10.1002/cncr.28732. [Epub ahead of print] PMID: 24771584 [PubMed - as supplied by publisher]
1.IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. Minniti G, Scaringi C, Arcella A, Lanzetta G, Di Stefano D, Scarpino S, Bozzao A, Pace A, Villani V, Salvati M, Esposito V, Giangaspero F, Enrici RM. J Neurooncol. 2014 Apr 20. [Epub ahead of print] PMID: 24748470 [PubMed - as supplied by publisher]
*以下の論文では、我々の抗体の論文3報が引用されているが、実際に使用されているのは、DianovaのH09抗体。
2.Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas. Arita H, Narita Y, Matsushita Y, Fukushima S, Yoshida A, Takami H, Miyakita Y, Ohno M, Shibui S, Ichimura K. Brain Tumor Pathol. 2014 Apr 19. [Epub ahead of print] PMID: 24748374 [PubMed - as supplied by publisher]
3.Glutamate as chemotactic fuel for diffuse glioma cells; are they glutamate suckers? van Lith SA, Navis AC, Verrijp K, Niclou SP, Bjerkvig R, Wesseling P, Tops B, Molenaar R, van Noorden CJ, Leenders WP. Biochim Biophys Acta. 2014 Apr 16. pii: S0304-419X(14)00040-7. doi: 10.1016/j.bbcan.2014.04.004. [Epub ahead of print] Review. PMID: 24747768 [PubMed - as supplied by publisher]
4. Rapid and sensitive intraoperative detection of mutations in the isocitrate dehydrogenase 1 and 2 genes during surgery for glioma. Kanamori M, Kikuchi A, Watanabe M, Shibahara I, Saito R, Yamashita Y, Sonoda Y, Kumabe T, Kure S, Tominaga T. J Neurosurg. 2014 Apr 18. [Epub ahead of print] PMID: 24745708 [PubMed - as supplied by publisher]
IDH2阻害剤のAG-221がIDH2変異を持つ進行血液がんに有効である可能性
イソクエン酸脱水素酵素(IDH)2阻害剤のAG-221が進行血液がんに有効である可能性が明らかとなった。用量増多フェーズI試験で有望な結果が得られたもの。4月4日から9日までサンディエゴで開催されているAmerican Association for Cancer Research(AACR2014)で、米Memorial Sloan Kettering Cancer CenterのEytan M.Stein氏によって発表された。
我々の論文がCancer Scienceにアクセプトされました。
Kaneko, Mika; Liu, Xing; Oki, Hiroharu; Ogasawara, Satoshi; Nakamura, Takuro; Saidoh, Noriko; Tsujimoto, Yuta; Matsuyama, Yuka; Uruno, Akira; Sugawara, Masato; Tsuchiya, Takashi; Yamakawa, Mitsunori; Yamamoto, Masayuki; Takagi, Michiaki; Kato, Yukinari
IDH2 mutation is frequently observed in giant cell tumor of bone.
Cancer Sci., in press
劉さんの論文(Cancer Med., 2014)が初めて、他の論文(総説)に引用されました。他にも、我々の2つの論文(NEON2012, CAS2014)が引用されています。
Biochim Biophys Acta. 2014 May 28. pii: S0304-419X(14)00049-3. doi: 10.1016/j.bbcan.2014.05.004. [Epub ahead of print]
The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation.
Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE.
Abstract
Mutations in isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) are key events in the development of glioma, acute myeloid leukemia (AML), chondrosarcoma, intrahepatic cholangiocarcinoma (ICC), and angioimmunoblastic T-cell lymphoma. They also cause D-2-hydroxyglutaric aciduria and Ollier and Maffucci syndromes. IDH1/2 mutations are associated with prolonged survival in glioma and in ICC, but not in AML. The reason for this is unknown. In their wild-type forms, IDH1 and IDH2 convert isocitrate and NADP+ to α-ketoglutarate (αKG) and NADPH. Missense mutations in the active sites of these enzymes induce a neo-enzymatic reaction wherein NADPH reduces αKG to D-2-hydroxyglutarate (D-2HG). The resulting D-2HG accumulation leads to hypoxia-inducible factor 1α degradation, changes in epigenetics and extracellular matrix homeostasis. Such mutations also imply less NADPH production capacity. Each of these effects could play a role in cancer formation. Here, we provide an overview of the literature and discuss which downstream molecular effects are likely to be the drivers of the oncogenic and survival-prolonging properties of IDH1/2 mutations. We discuss interactions between mutant IDH1/2 inhibitors and conventional therapies. Understanding of the biochemical consequences of IDH1/2 mutations in oncogenesis and survival prolongation will yield valuable information for rational therapy design: it will tell us which oncogenic processes should be blocked and which "survivalogenic" effects should be retained.
Nature. 2014 Jul 2. doi: 10.1038/nature13441. [Epub ahead of print]
Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer.
Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, Miltiadous O, Walesky C, Deshpande V, Zhu AX, Hezel AF, Yen KE, Straley KS, Travins J, Popovici-Muller J, Gliser C, Ferrone CR, Apte U, Llovet JM, Wong KK, Ramaswamy S, Bardeesy N.
Abstract
Mutations in isocitrate dehydrogenase 1 (IDH1) and IDH2 are among the most common genetic alterations in intrahepatic cholangiocarcinoma (IHCC), a deadly liver cancer. Mutant IDH proteins in IHCC and other malignancies acquire an abnormal enzymatic activity allowing them to convert α-ketoglutarate (αKG) to 2-hydroxyglutarate (2HG), which inhibits the activity of multiple αKG-dependent dioxygenases, and results in alterations in cell differentiation, survival, and extracellular matrix maturation. However, the molecular pathways by which IDH mutations lead to tumour formation remain unclear. Here we show that mutant IDH blocks liver progenitor cells from undergoing hepatocyte differentiation through the production of 2HG and suppression of HNF-4α, a master regulator of hepatocyte identity and quiescence. Correspondingly, genetically engineered mouse models expressing mutant IDH in the adult liver show an aberrant response to hepatic injury, characterized by HNF-4α silencing, impaired hepatocyte differentiation, and markedly elevated levels of cell proliferation. Moreover, IDH and Kras mutations, genetic alterations that co-exist in a subset of human IHCCs, cooperate to drive the expansion of liver progenitor cells, development of premalignant biliary lesions, and progression to metastatic IHCC. These studies provide a functional link between IDH mutations, hepatic cell fate, and IHCC pathogenesis, and present a novel genetically engineered mouse model of IDH-driven malignancy.
Nature. 2014 Jun 25. doi: 10.1038/nature13387. [Epub ahead of print]
A vaccine targeting mutant IDH1 induces antitumour immunity.
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, Keil M, Bals J, Rauschenbach K, Grabowska AK, Vogler I, Diekmann J, Trautwein N, Eichmuller SB4, Okun J, Stevanovi? S, Riemer AB, Sahin U, Friese MA, Beckhove P, von Deimling A, Wick W, Platten M.
Abstract
Monoallelic point mutations of isocitrate dehydrogenase type 1 (IDH1) are an early and defining event in the development of a subgroup of gliomas and other types of tumour. They almost uniformly occur in the critical arginine residue (Arg?132) in the catalytic pocket, resulting in a neomorphic enzymatic function, production of the oncometabolite 2-hydroxyglutarate (2-HG), genomic hypermethylation, genetic instability and malignant transformation. More than 70% of diffuse grade II and grade III gliomas carry the most frequent mutation, IDH1(R132H) (ref. 3). From an immunological perspective, IDH1(R132H) represents a potential target for immunotherapy as it is a tumour-specific potential neoantigen with high uniformity and penetrance expressed in all tumour cells. Here we demonstrate that IDH1(R132H) contains an immunogenic epitope suitable for mutation-specific vaccination. Peptides encompassing the mutated region are presented on major histocompatibility complexes (MHC) class II and induce mutation-specific CD4+ T-helper-1 (TH1) responses. CD4+ TH1 cells and antibodies spontaneously occurring in patients with IDH1(R132H)-mutated gliomas specifically recognize IDH1(R132H). Peptide vaccination of mice devoid of mouse MHC and transgenic for human MHC class I and II with IDH1(R132H) p123-142 results in an effective MHC class II-restricted mutation-specific antitumour immune response and control of pre-established syngeneic IDH1(R132H)-expressing tumours in a CD4+ T-cell-dependent manner. As IDH1(R132H) is present in all tumour cells of these slow-growing gliomas, a mutation-specific anti-IDH1(R132H) vaccine may represent a viable novel therapeutic strategy for IDH1(R132H)-mutated tumours.
Cancer Res. 2014 Jul 17. pii: canres.0924.2014. [Epub ahead of print]
Mutant IDH1-driven cellular transformation increases RAD51-mediated homologous recombination and temozolomide resistance.
Ohba S, Mukherjee J, See WL, Pieper RO.
Abstract
Isocitrate dehydrogenase 1 (IDH1) mutations occur in most lower-grade glioma, and not only drive gliomagenesis but are associated with longer patient survival and improved response to temozolomide (TMZ). To investigate the possible causative relationship between these events, we introduced wild-type (WT) or mutant IDH1 into immortalized, untransformed human astrocytes, then monitored transformation status and TMZ response. TMZ-sensitive parental cells exhibited DNA damage (gamma-H2AX foci) and a prolonged G2 cell cycle arrest beginning 3 days after TMZ (100μM, 3hr) exposure and persisting for greater than 4 days. The same cells transformed by expression of mutant IDH1 exhibited a comparable degree of DNA damage and cell cycle arrest, but both events resolved significantly faster in association with increased, rather than decreased, clonogenic survival. The increases in DNA damage processing, cell cycle progression, and clonogenicity were unique to cells transformed by mutant IDH1, and were not noted in cells transformed by WT IDH1 or an oncogenic form (V12H) of Ras. Similarly these effects were not noted following introduction of mutant IDH1 into Ras-transformed cells or established GBM cells. They were, however, associated with increased homologous recombination and could be reversed by the genetic or pharmacologic suppression of the homologous recombination DNA repair protein RAD51. These results show that mutant IDH1 drives a unique set of transformative events that indirectly enhance homologous recombination and facilitate repair of TMZ-induced DNA damage and TMZ resistance. The results also suggest that inhibitors of HR may be a viable means to enhance TMZ response in IDH1 mutant glioma.
我々の抗体が複数引用されています。
Brain Tumor Pathol. 2014 Jul 10. [Epub ahead of print]
IDH1/2 mutation detection in gliomas.
Arita H, Narita Y, Yoshida A, Hashimoto N, Yoshimine T, Ichimura K.
Abstract
Somatic mutations of isocitrate dehydrogenase 1 and 2 (IDH1/2) are strongly associated with pathological subtypes, genetic profiles, and clinical features in gliomas. The IDH1/2 status is currently regarded as one of the most important molecular markers in gliomas and should be assessed accurately and robustly. However, the methods used for IDH1/2 testing are not fully standardized. The purpose of this paper is to review the clinical significance of IDH1/2 mutations and the methods used for IDH1/2 testing. The optimal method for IDH1/2 testing varies depending on a number of factors, including the purpose, sample types, sample number, or laboratory equipment. It is therefore important to acknowledge the advantages and disadvantages of each method.
Am J Surg Pathol. 2014 Aug;38(8):1147-56. doi: 10.1097/PAS.0000000000000239.
Isocitrate Dehydrogenase-1 Is Mutated in Inflammatory Bowel Disease-associated Intestinal Adenocarcinoma With Low-grade Tubuloglandular Histology but Not in Sporadic Intestinal Adenocarcinoma.
Hartman DJ, Binion D, Regueiro M, Schraut W, Bahary N, Sun W, Nikiforova M, Pai RK.
Abstract
The underlying molecular alterations in chronic idiopathic inflammatory bowel disease-associated intestinal adenocarcinoma remain largely unknown. Somatic IDH mutations are often seen in gliomas and myeloid leukemia but have also been recently reported in a subset of other neoplasms. We analyzed a series of intestinal adenocarcinomas with (n=23) and without (n=39) associated chronic idiopathic inflammatory bowel disease treated at our institution for IDH1 and IDH2 mutations and correlated the clinicopathologic findings with mutation status. Compared with intestinal adenocarcinomas not associated with inflammatory bowel disease, adenocarcinomas associated with inflammatory bowel disease more frequently demonstrated IDH mutations (13% vs. 0%, P=0.047). All IDH mutations were identified in IDH1 and resulted in substitution of arginine by cysteine at position 132 (p.R132C, c.394C>T). IDH1 mutations were frequently (66%) associated with concurrent KRAS mutations (p.G12D, c.35G>A). IDH1-mutated intestinal adenocarcinomas were seen in the setting of both Crohn disease and ulcerative colitis and were located in both the ileum and colon. Compared with IDH1-negative inflammatory bowel disease-associated adenocarcinoma, IDH1-positive adenocarcinomas more frequently demonstrated tubuloglandular histology (100% vs. 25%, P=0.032) and were more frequently associated with precursor lesions exhibiting serrated morphology (66% vs. 6%, P=0.034). IDH1 mutations were also identified in the precursor dysplastic lesions associated with IDH1-positive adenocarcinomas. In conclusion, we demonstrate that IDH1 mutations are occasionally identified in inflammatory bowel disease-associated intestinal adenocarcinoma but not in intestinal adenocarcinoma not associated with inflammatory bowel disease. In addition, IDH1-mutated intestinal adenocarcinoma is associated with a characteristic low-grade tubuloglandular histology and often harbors concurrent KRAS mutations. Identification of patients with IDH1-mutated intestinal adenocarcinoma may become clinically important as new therapies emerge that target tumors that harbor IDH mutations.
Acta Neuropathol. 2014 Aug 21. [Epub ahead of print]
Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma.
Sahm F, Reuss D, Koelsche C, Capper D, Schittenhelm J, Heim S, Jones DT, Pfister SM, Herold-Mende C, Wick W, Mueller W, Hartmann C, Paulus W, von Deimling A.
Abstract
Astrocytoma and oligodendroglioma are histologically and genetically well-defined entities. The majority of astrocytomas harbor concurrent TP53 and ATRX mutations, while most oligodendrogliomas carry the 1p/19q co-deletion. Both entities share high frequencies of IDH mutations. In contrast, oligoastrocytomas (OA) appear less clearly defined and, therefore, there is an ongoing debate whether these tumors indeed constitute an entity or whether they represent a mixed bag containing both astrocytomas and oligodendrogliomas. We investigated 43 OA diagnosed in different institutions employing histology, immunohistochemistry and in situ hybridization addressing surrogates for the molecular genetic markers IDH1R132H, TP53, ATRX and 1p/19q loss. In all but one OA the combination of nuclear p53 accumulation and ATRX loss was mutually exclusive with 1p/19q co-deletion. In 31/43 OA, only alterations typical for oligodendroglioma were observed, while in 11/43 OA, only indicators for mutations typical for astrocytomas were detected. A single case exhibited a distinct pattern, nuclear expression of p53, ATRX loss, IDH1 mutation and partial 1p/19q loss. However, this was the only patient undergoing radiotherapy prior to surgery, possibly contributing to the acquisition of this uncommon combination. In OA with oligodendroglioma typical alterations, the portions corresponding to astrocytic part were determined as reactive, while in OA with astrocytoma typical alterations the portions corresponding to oligodendroglial differentiation were neoplastic. These data provide strong evidence against the existence of an independent OA entity.
加藤が執筆した総説がPubMedに掲載されました。Table1が一カ所間違っていましたので、以下のように訂正します。(MsMab-1はR132Gを免疫しましたので、R132Gに対する反応はOriginalとなります。)
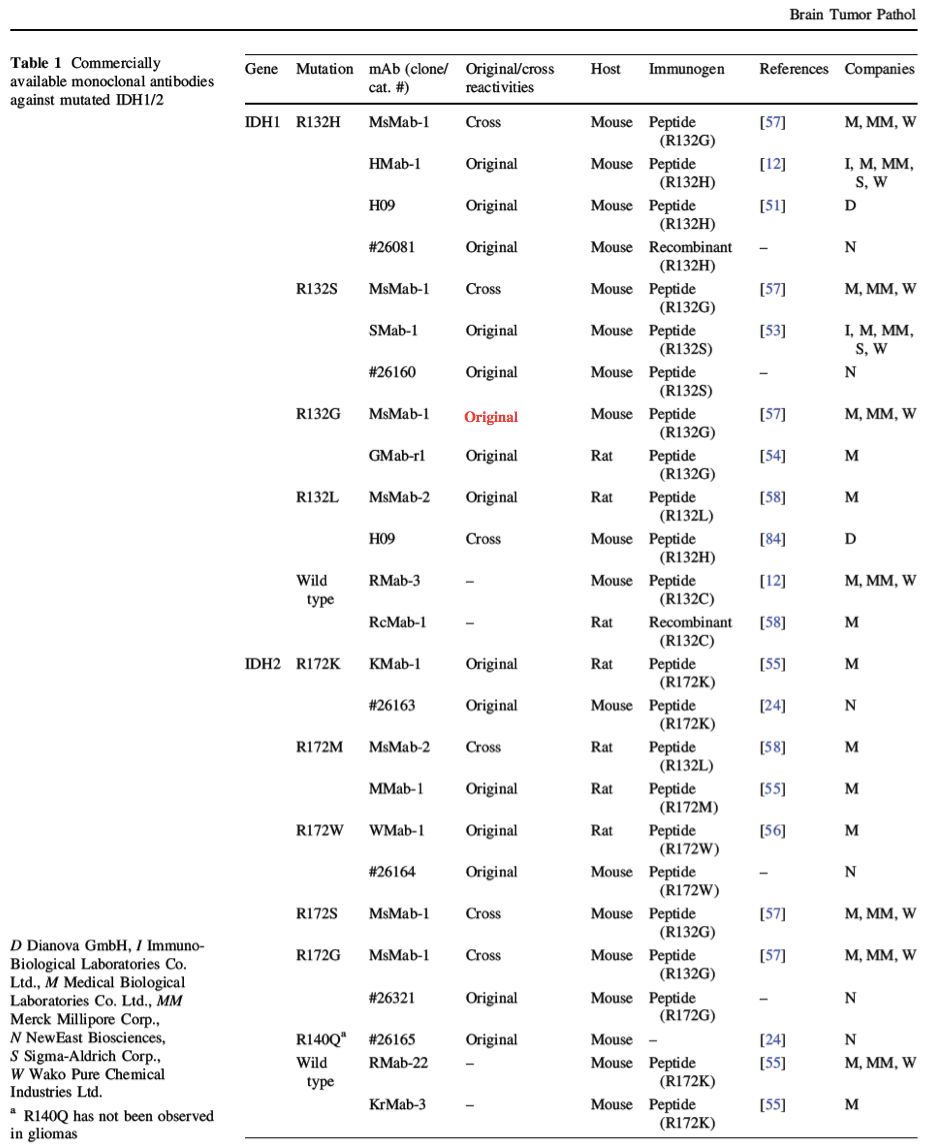
加藤が執筆した総説がPubMedに掲載されました。
Brain Tumor Pathol. 2014 Oct 17. [Epub ahead of print]
Specific monoclonal antibodies against IDH1/2 mutations as diagnostic tools for gliomas.
Kato Y.
Abstract
Mutations of isocitrate dehydrogenase 1/2 (IDH1/2) have been reported in gliomas and other types of tumors, such as acute myeloid leukemias, cartilaginous tumors, intrahepatic cholangiocarcinomas, osteosarcomas, and giant cell tumors of bone. In gliomas, IDH mutations uniformly occur in the functionally critical arginine 132 residue (R132) of IDH1 and arginine 172 residue (R172) of IDH2. IDH1 and IDH2 catalyze the oxidative carboxylation of isocitrate to α-ketoglutarate (α-KG) in the cytosol and mitochondria, respectively. In contrast, mutated IDH1/2 proteins possess a neomorphic enzymatic function that changes α-KG into the oncometabolite, R(-)-2-hydroxyglutarate, resulting in genomic hypermethylation, histone methylation, genetic instability, and malignant transformation. To date, several monoclonal antibodies (mAbs) specific for IDH1/2 mutations such as anti-IDH1-R132H mAbs (clone H09, clone IMab-1, and clone HMab-1) or an anti-IDH1-R132S mAb (clone SMab-1) have been established. Furthermore, one of multi-specific mAbs, MsMab-1, recognizes IDH1 mutants (R132H, R132S, R132G) and IDH2 mutants (R172S, R172G), which are observed in gliomas. Another mAb, MsMab-2, recognizes IDH1-R132L and IDH2-R172M. These IDH1/2 mutation-specific mAbs are useful for the immunohistochemical determination of IDH1/2 mutation-bearing gliomas.
PMID: 25324168 [PubMed - as supplied by publisher]
J Biol Chem. 2014 Nov 12. pii: jbc.M114.608497. [Epub ahead of print]
PMID: 25391653 [PubMed - as supplied by publisher](http://www.ncbi.nlm.nih.gov/pubmed/25388165)
Selective Inhibition of Mutant Isocitrate Dehydrogenase 1 (IDH1) via Disruption of a Metal Binding Network by an Allosteric Small Molecule.
Deng G, Shen J, Yin M, McManus J, Mathieu M, Gee P, He T, Shi C, Bedel O, McLean LR, Le-Strat F, Zhang Y, Marquette JP, Gao Q, Zhang B, Rak A, Hoffmann D, Rooney E, Vassort A, Englaro W, Li Y, Patel V, Adrian F, Gross S, Wiederschain D, Cheng H, Licht S.
Abstract
Cancer-associated point mutations in isocitrate dehydrogenase 1 and 2 (IDH1, IDH2) confer a neomorphic enzymatic activity: the reduction of alpha-ketoglutarate (αKG) to D-2-hydroxyglutaric acid (2HG), which is proposed to act as an oncogenic metabolite by inducing hypermethylation of histones and DNA. While selective inhibitors of mutant IDH1 and IDH2 have been identified and are currently under investigation as potential cancer therapeutics, the mechanistic basis for their selectivity is not yet well-understood. A high-throughput screen for selective inhibitors of IDH1 bearing the oncogenic mutation R132H identified Compound 1, a bis-imidazole phenol that inhibits 2HG production in cells. We investigated the mode of inhibition of Compound 1 and a previously published IDH1 mutant inhibitor with a different chemical scaffold. Steady-state kinetics and biophysical studies show that both of these compounds selectively inhibit mutant IDH1 by binding to an allosteric site, and that inhibition is competitive with respect to Mg2+. A crystal structure of Compound 1 complexed with R132H IDH1 indicates that the inhibitor binds at the dimer interface and makes a direct contact with a residue involved in binding of the catalytically essential divalent cation. These results show that targeting a divalent cation binding residue can enable selective inhibition of mutant IDH1, and suggest that differences in magnesium binding between wild-type and mutant enzymes may contribute to the inhibitors' selectivity for the mutant enzyme.
PMID:
25391653
[PubMed - as supplied by publisher]
ドイツApogenix社、膠芽腫に対するCD95受容体-Fc融合蛋白のフェーズII結果を発表(https://bio.nikkeibp.co.jp/article/news/20141104/180039/)
Apogenix社の旗艦製品であるAPG101 は、ヒト融合蛋白質で、CD95受容体の細胞外ドメインと、IgG抗体のFc領域から構成される。CD95リガンドと、 CD95 受容体の相互作用が、細胞内シグナリングパスウェイを活性化させる。それが、浸潤性増殖や、膠芽腫細胞などの腫瘍細胞の移転を促進する。
我々の開発したHMab-1抗体(anti-IDH1-R132H mAb)が使われています。ミリポアから販売されているHMab-1が使われていますが、販売されている商品が論文で使用されたのは初めてです。
Proc Natl Acad Sci U S A. 2014 Jul 29;111(30):11121-6. doi: 10.1073/pnas.1404724111. Epub 2014 Jun 30.
Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery.
Santagata S, Eberlin LS, Norton I, Calligaris D, Feldman DR, Ide JL, Liu X, Wiley JS, Vestal ML, Ramkissoon SH, Orringer DA, Gill KK, Dunn IF, Dias-Santagata D, Ligon KL, Jolesz FA, Golby AJ, Cooks RG, Agar NY.
Abstract
For many intraoperative decisions surgeons depend on frozen section pathology, a technique developed over 150 y ago. Technical innovations that permit rapid molecular characterization of tissue samples at the time of surgery are needed. Here, using desorption electrospray ionization (DESI) MS, we rapidly detect the tumor metabolite 2-hydroxyglutarate (2-HG) from tissue sections of surgically resected gliomas, under ambient conditions and without complex or time-consuming preparation. With DESI MS, we identify isocitrate dehydrogenase 1-mutant tumors with both high sensitivity and specificity within minutes, immediately providing critical diagnostic, prognostic, and predictive information. Imaging tissue sections with DESI MS shows that the 2-HG signal overlaps with areas of tumor and that 2-HG levels correlate with tumor content, thereby indicating tumor margins. Mapping the 2-HG signal onto 3D MRI reconstructions of tumors allows the integration of molecular and radiologic information for enhanced clinical decision making. We also validate the methodology and its deployment in the operating room: We have installed a mass spectrometer in our Advanced Multimodality Image Guided Operating (AMIGO) suite and demonstrate the molecular analysis of surgical tissue during brain surgery. This work indicates that metabolite-imaging MS could transform many aspects of surgical care.
PMID:
24982150
Acta Neuropathol. 2014 Nov 27. [Epub ahead of print]
ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma.
Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V, Mittelbronn M, Schittenhelm J, Herold-Mende C, Unterberg A, Platten M, Weller M, Wick W, Pfister SM, von Deimling A.
Abstract
Diffuse gliomas are represented in the 2007 WHO classification as astrocytomas, oligoastrocytomas and oligodendrogliomas of grades II and III and glioblastomas WHO grade IV. Molecular data on these tumors have a major impact on prognosis and therapy of the patients. Consequently, the inclusion of molecular parameters in the WHO definition of brain tumors is being planned and has been forwarded as the "ISN-Haarlem" consensus. We, here, analyze markers of special interest including ATRX, IDH and 1p/19q codeletion in a series of 405 adult patients. Among the WHO 2007 classified tumors were 152 astrocytomas, 61 oligodendrogliomas, 63 oligoastrocytomas and 129 glioblastomas. Following the concepts of the "ISN-Haarlem", we rediagnosed the series to obtain "integrated" diagnoses with 155 tumors being astrocytomas, 100 oligodendrogliomas and 150 glioblastomas. In a subset of 100 diffuse gliomas from the NOA-04 trial with long-term follow-up data available, the "integrated" diagnosis had a significantly greater prognostic power for overall and progression-free survival compared to WHO 2007. Based on the "integrated" diagnoses, loss of ATRX expression was close to being mutually exclusive to 1p/19q codeletion, with only 2 of 167 ATRX-negative tumors exhibiting 1p/19q codeletion. All but 4 of 141 patients with loss of ATRX expression and diffuse glioma carried either IDH1 or IDH2 mutations. Interestingly, the majority of glioblastoma patients with loss of ATRX expression but no IDH mutations exhibited an H3F3A mutation. Further, all patients with 1p/19 codeletion carried a mutation in IDH1 or IDH2. We present an algorithm based on stepwise analysis with initial immunohistochemistry for ATRX and IDH1-R132H followed by 1p/19q analysis followed by IDH sequencing which reduces the number of molecular analyses and which has a far better association with patient outcome than WHO 2007.
Virchows Arch. 2014 Nov 29. [Epub ahead of print]
Isocitrate dehydrogenase 1 mutations (IDH1) and p16/CDKN2A copy number change in conventional chondrosarcomas.
Amary MF, Ye H, Forbes G, Damato S, Maggiani F, Pollock R, Tirabosco R, Flanagan AM.
Abstract
To determine whether IDH1 mutations are present in primary and relapsed (local and distal) conventional central chondrosarcomas; and secondly, to assess if loss of p16/CDKN2A is associated with tumour grade progression, 102 tumour samples from 37 patients, including material from presenting and relapse events, were assessed. All wild-type cases for IDH1 R132 substitutions were also tested for IDH2 R172 and R140 alterations. The primary tumour and the most recent relapse sample were tested for p16/CDKN2A by interphase fluorescence in situ hybridisation. An additional 120 central cartilaginous tumours from different patients were also tested for p16/CDKN2A copy number. The study shows that if an IDH1 mutation were detected in a primary central chondrosarcoma, it is always detected at the time of presentation, and the same mutation is detected in local recurrences and metastatic events. We show that p16/CDKN2A copy number variation occurs subsequent to the IDH1 mutation, and confirm that p16/CDKN2A copy number variation occurs in 75 % of high grade central chondrosarcomas, and not in low grade cartilaginous tumours. Finally, p16/CDKN2A copy number variation is seen in both the IDH1 wild-type and mutant cartilaginous central tumours.