
・Amano I, Imaizumi Y, Kaji C, Kojima H, Sawa Y.
Expression of podoplanin and classical cadherins in salivary gland epithelial cells of klotho-deficient mice.
Acta Histochem Cytochem. 2011 Dec 28;44(6):267-76. Epub 2011 Nov 5.
Abstract
We have recently shown that salivary gland myoepithelial cells express podoplanin. Podoplanin indirectly binds the actin filament network which links classical cadherins. The study here is aimed to investigate the expression of podoplanin and cadherins on salivary gland myoepithelial cells and the changes in the aging cells using klotho-deficient (kl/kl) mice. The submandibular glands of kl/kl mouse lack granular ducts which express klotho in wild type mice, suggesting that klotho may be a gene responsible for granular duct development. Although aging resulted in growth suppression of myoepithelial cells because of the sparse distribution of the cells in kl/kl mouse salivary glands, the expression of podoplanin and E-cadherin was shown in aging myoepithelial cells. It is thought that podoplanin participates in the actin-E-cadherin networks which are maintained in aging myoepithelial cells. It was also shown that granular ducts were filled with P-cadherin, and that the P-cadherin amount was larger in the wild type mouse submandibular glands than in the sublingual and parotid glands of wild type mouse, and in the submandibular glands of kl/kl mouse. These findings suggest that the granular duct is an organ secreting soluble P-cadherin into the saliva.
・<The expression of podoplanin and classical cadherins in the brain.>がJournal of Anatomyにアクセプトされました。
・Ordonez NG.
Podoplanin expression in wound and hyperproliferative psoriatic epidermis: Mesotheliomas with small cell features: report of eight cases.
Mod Pathol. 2012 Jan 6. doi: 10.1038/modpathol.2011.202. [Epub ahead of print]
Abstract
Mesotheliomas with small cell morphology are rare and only one study of such cases has been published. As a result of their rare occurrence, some investigators have cast doubt on the existence of such a histologic variant of mesothelioma. This investigator reports a series of eight cases of epithelioid mesothelioma with small cell features, all of which originated in the pleura. Seven of the patients were men and one was a woman. Four patients had a history of asbestos exposure. Histologically, four of the mesotheliomas were epithelioid and four biphasic. The proportion of small cells seen in these cases constituted 80 to 100% of the tumor included in the biopsy material and 15 to 20% of the tumor present in the pneumonectomy specimens. Immunoreactivity for calretinin, keratin 5/6, keratin 7, pan-keratin, WT1, podoplanin, and mesothelin was seen in all cases tested for these markers. All of the cases were negative for MOC-31, Ber-EP4, CEA, CD15, TAG-72, TTF-1, chromogranin A, synaptophysin, CD99, and desmin. The mean survival of the six patients for whom this information was available was 8.2 months. It is important for pathologists to be aware that mesotheliomas can present small cell features and, because of this, they can be confused with other malignancies that can exhibit similar morphology. The value of immunohistochemistry in the differential diagnosis of these tumors is discussed.
・我々の<動脈硬化におけるポドプラニン発現>の論文がThrombosis Researchにアクセプトされました。宮崎大学との共同研究です。
Neuro Oncol. 2012 Mar 6. [Epub ahead of print]
Expression of podoplanin in human astrocytic brain tumors is controlled by the PI3K-AKT-AP-1 signaling pathway and promoter methylation.
Peterziel H, Muller J, Danner A, Barbus S, Liu HK, Radlwimmer B, Pietsch T, Lichter P, Schutz G, Hess J, Angel P.
Abstract
Recently, we found strong overexpression of the mucin-type glycoprotein podoplanin (PDPN) in human astrocytic brain tumors, specifically in primary glioblastoma multiforme (GB). In the current study, we show an inverse correlation between PDPN expression and PTEN levels in primary human GB and glioma cell lines, and we report elevated PDPN protein levels in the subventricular zone of brain tissue sections of PTEN-deficient mice. In human glioma cells lacking functional PTEN, reintroduction of wild-type PTEN, inhibition of the PTEN downstream target protein kinase B/AKT, or interference with transcription factor AP-1 function resulted in efficient downregulation of PDPN expression. In addition, we observed hypoxia-dependent PDPN transcriptional control and demonstrated that PDPN expression is subject to negative transcriptional regulation by promoter methylation in human GB and in glioma cell lines. Treatment of PTEN-negative glioma cells with demethylating agents induced expression of PDPN. Together, our findings show that increased PDPN expression in human GB is caused by loss of PTEN function and activation of the PI3K-AKT-AP-1 signaling pathway, accompanied by epigenetic regulation of PDPN promoter activity. Silencing of PDPN expression leads to reduced proliferation and migration of glioma cells, suggesting a functional role of PDPN in glioma progression and malignancy. Thus, specific targeting of PDPN expression and/or function could be a promising strategy for the treatment of patients with primary GB.
Chest. 2012 Feb 2. [Epub ahead of print]
Prognostic impact of cancer-associated stromal cells in stage I lung adenocarcinoma patients.
Ito M, Ishii G, Nagai K, Maeda R, Nakano Y, Ochiai A.
Abstract:
BACKGROUND:
The tumor microenvironment, of which cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs) are the major cellular components, plays an important role in tumor progression. This study evaluated the significance of podoplanin-positive CAFs and CD204-positive TAMs, which may reflect tumor-promoting CAFs and TAMs, as risk factors for recurrence in patients with stage I lung adenocarcinoma.
METHODS:
The expression of podoplanin in CAFs and CD204 in TAMs was analyzed by immunohistochemistry in 304 stage I lung adenocarcinoma patients who underwent surgical resection between September 1992 and July 2004. The recurrence-free proportion (RFP) was estimated using the Kaplan-Meier method.
RESULTS:
The presence of podoplanin-positive CAFs and the higher number of CD204-positive TAMs were associated with a lower 5-year RFP (p < 0.001 and p = 0.001, respectively). Podoplanin-positive CAFs was shown to be an independently statistically significant risk factor for recurrence with the highest hazard ratio (HR 3.474, p = 0.029, by multivariate Cox proportional hazards model). According to subgroup analyses combining podoplanin-positive CAFs and other independent risk factors (visceral pleural invasion and intratumoral vascular invasion), the 5-year RFPs were 95.6%, 92.3%, 80.5%, and 30.3% (p = 0.294, p = 0.067, and p < 0.001) for patients with zero, one, two, or three risk factors, respectively.
CONCLUSION:
Podoplanin-positive CAFs was the most powerful independent risk factor for recurrence in patients with stage I lung adenocarcinoma. Podoplanin-positive CAFs may be useful for identifying patients with a high risk of recurrence who might benefit from adjuvant chemotherapy.
・「新規血小板凝集促進因子Aggrusを標的 とした分子標的治療薬の創製 」 【研究期間】H21~H25 :中間報告書(藤田直也先生)
和光純薬のBioWindowに、我々の抗体(NZ-1.2とPMab-1)が掲載されました(P. 14)。
Oral Oncol. 2012 Apr 21. [Epub ahead of print]
Expression of podoplanin and ABCG2 in oral erythroplakia correlate with oral cancer development.
Feng JQ, Mi JG, Wu L, Ma LW, Shi LJ, Yang X, Liu W, Zhang CP, Zhou ZT.
Department of Preventive Dentistry, Shanghai Municipal Hospital for Oral Health, Shanghai, China; Shanghai Key Laboratory of Stomatology, Department of Oral Mucosal Diseases, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai, China.
Abstract
Oral erythroplakia (OE) is a notoriously aggressive oral premalignant lesion with a high tendency to oral cancer development, but it's biological behavior is largely unknown. The objective of the current study was to determine podoplanin and ABCG2 immunoexpression in OE and both correlation to malignant transformation of OE. In a retrospective follow-up study, the expression patterns of podoplanin and ABCG2 were determined using immunohistochemistry in samples from 34 patients with OE, including patients with untransformed lesions (n=17) and patients with malignant transformed lesions (n=17). Podoplanin and ABCG2 expression was observed in 15 (44.1%) and 21 (61.8%) of 34 patients, respectively. Multivariate analysis revealed that podoplanin and ABCG2 expression was associated with 6.31-fold (95% confidence interval [CI], 1.02-38.92; P=0.047) and 14.39-fold (95% CI, 2.02-102.29; P=0.008) increased the risk of transformation, respectively. Point prevalence analysis revealed that 90.9% (95% CI, 70.7-100) of the patient with both podoplanin and ABCG2 positivity developed oral cancer. Collectively, our data indicated that the expression patterns of podoplanin and ABCG2 in OE were associated with oral cancer development, suggesting that podoplanin and ABCG2 may be valuable predictors for evaluating oral cancer risk.
Virchows Arch. 2012 Apr 4. [Epub ahead of print]
Nonsebaceous lymphadenoma of salivary glands: proposed development from intraparotid lymph nodes and risk of misdiagnosis.
Weiler C, Agaimy A, Zengel P, Zenk J, Kirchner T, Ihrler S.
Institute of Pathology, Ludwig Maximilian University, Thalkirchnerstrase 36, D-80337, Munchen, Germany, christoph.weiler@lrz.uni-muenchen.de.
Abstract
Nonsebaceous lymphadenoma (NSLA) is a rare benign salivary gland tumor composed of lymphoid and epithelial components. By definition, the epithelial component lacks sebaceous differentiation and instead displays a wide range of histological differentiation. In this study, we have collected nine cases of NSLA to characterize their histological and immunohistochemical profiles. The samples were histologically reviewed and immunohistochemical stains for CK5/6, CK7, CK14, CK18, p63, and Ki67 performed. Patients were six males and three females (mean age, 50 years). All tumors were located in the parotid gland and showed intimate intermingling of lymphoid tissue with islands or strands of epithelium with a wide spectrum of histological differentiation. The immunohistochemical profiles mirrored the epithelial differentiation; hence, areas with basaloid or lymphoepithelial differentiation strongly expressed CK5/6, CK14, and p63, while areas with ductal differentiation showed strong positivity for CK18/CK7 and CK5/6/CK14/p63 in luminal and basal cell layers, respectively. A hilus structure with salivary inclusions or D2-40 (podoplanin)-positive marginal sinus was identifiable in four and nine of the cases, respectively, supporting origin within intra-/periparotid lymph nodes. Six cases were initially misdiagnosed as other benign (n?=?4) or malignant tumors (n?=?2). Our study on the second largest series of NSLA reported to date provides strong evidence that NSLA belongs to the group of salivary gland tumors that pathogenetically develop from embryonic salivary gland inclusions in intra-/periparotid lymph nodes. Knowledge of the wide histological spectrum of this rare and presumably underreported tumor is important in order to avoid misdiagnosis, particularly as malignant tumor.
Oral Dis. 2012 Mar 3. doi: 10.1111/j.1601-0825.2012.01927.x. [Epub ahead of print]
Podoplanin expression in oral leukoplakia: prognostic value and clinicopathological implications.
Kreppel M, Kreppel B, Drebber U, Wedemayer I, Rothamel D, Zoller J, Scheer M.
Department for Oral and Cranio-Maxillo and Facial Plastic Surgery, University of Cologne, Cologne, Germany Centre of Integrated Oncology (CIO) Cologne-Bonn, Cologne, Germany Department of Pathology, University of Cologne, Cologne, Germany.
Abstract
Oral Diseases (2012) Objectives:? Current clinicopathological parameters cannot predict the risk of malignant transformation in oral leukoplakia sufficiently. Recent studies have shown that podoplanin is expressed in oral cancer and precancerous lesions. The aim of our study was to assess whether podoplanin expression in pretreatment biopsies could serve as a biomarker to predict the risk of malignant transformation in patients with oral leukoplakia. Materials and Methods:? In this retrospective study, podoplanin expression was analysed in 60 patients with previously untreated oral leukoplakia by immunohistochemistry. We investigated the associations between podoplanin expression and various clinicopathological variables including oral cancer-free survival (OCFS) and the SIN-classification. Results:? The chi-square-test revealed that high expression of podoplanin in pretreatment biopsies was associated with malignant transformation (P?=?0.003) and increasing SIN-classification (P?=?0.009). In univariate analysis, podoplanin expression in oral leukoplakia had a significant impact on OCFS (P?=?0.009). The 5-year OCFS rate decreased from 100% for patients with no podoplanin expression to 41.7% for patients with the highest level of podoplanin expression. Conclusion:? Although podoplanin expression and the SIN-classification served as factors to predict malignant transformation in patients with oral leukoplakia in univariate analysis, no significant impact was found for both factors in multivariate analysis.
Expression of podoplanin in nevoid Basal cell carcinoma syndrome-associated keratocystic odontogenic tumours.
Friedrich RE, Scheuer HA, Zustin J.
Anticancer Res. 2012 May;32(5):2125-7.
Expression of podoplanin in primary and metastatic poorly differentiated and undifferentiated carcinomas of the head and neck.
Friedrich RE, Bartel-Friedrich S, Hagel C.
Anticancer Res. 2012 May;32(5):2019-22.
Mol Med Report. 2012 May 9. doi: 10.3892/mmr.2012.911. [Epub ahead of print]
Expression of podoplanin in salivary gland adenoid cystic carcinoma and its association with distant metastasis and clinical outcomes.
Wu HM, Ren GX, Wang LZ, Zhang CY, Chen WT, Guo W.
Department of Oral and Maxillofacial - Head and Neck Oncology, Ninth People's Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, P.R. China
Abstract
Distant metastasis is a common cause of mortality in patients with salivary gland adenoid cystic carcinoma (SACC). However, presently, the development of distant metastasis is unable to be predicted in clinical practice. Recent studies have shown that overexpression of podoplanin is associated with metastasis and survival in patients with several cancer types. The purpose of the present study was to determine whether podoplanin is overexpressed in SACC and whether such overexpression is associated with distant metastasis and survival. Podoplanin expression was determined using immunohistochemistry (IHC) in tumors from 40 SACC patients. The expression status was analyzed in regards to patient clinicopatholo-gical parameters and survival rates. Overexpression of podoplanin was detected in 13 (32.5%) of the 40 tumors. Overexpression was significantly associated with disease-free survival (P=0.025) and distant metastasis (P=0.015), although it was not associated with recurrence and overall survival. In conclusion, podoplanin is overexpressed in a subset of SACCs and may be a biomarker predicting distant metastasis in patients with SACC.
*沢先生のラボの論文が2報引用されている。
Hata M, Amano I, Tsuruga E, Kojima H and Sawa Y: Immunoelectron microscopic study of podoplanin localization in mouse salivary gland myoepithelium. Acta Histochem Cytochem 43: 77-82, 2010.
Hata M, Ueki T, Sato A, Kojima H and Sawa Y: Expression of podoplanin in the mouse salivary glands. Arch Oral Biol 53: 835-841, 2008.
Biochem Biophys Res Commun. 2012 May 7. [Epub ahead of print]
Tumor promoting effect of podoplanin-positive fibroblasts is mediated by enhanced RhoA activity.
Ito S, Ishii G, Hoshino A, Hashimoto H, Neri S, Kuwata T, Higashi M, Nagai K, Ochiai A.
Laboratory of Cancer Biology, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, Kashiwa, Chiba, Japan; Pathology Division, Research Center for Innovative Oncology, National Cancer Center Hospital East, Kashiwa, Chiba, Japan.
Abstract
There is growing evidence that stromal fibroblasts can promote tumor progression via several mechanisms. We previously reported that podoplanin (PDPN) expressed on stromal fibroblasts is functionally protein responsible for the promotion of tumor formation in mouse subcutaneous tissue. The purpose of the present study was to reveal the molecular mechanism by which PDPN on stromal fibroblasts promotes tumor formation. The subcutaneous co-injection of the human lung adenocarcinoma cell line A549 and human fibroblasts (hFbs) overexpressing wild-type podoplanin (WT-PDPN) promoted subcutaneous tumor formation, compared with the co-injection of A549 and control hFbs (64% vs 21%). On the other hand, hFbs expressing PDPN mutant in which the cytoplasmic domain of PDPN was deleted (PDPN-Del.IC), resulted in a relatively lower level of tumor formation (33%). Since PDPN reportedly regulates RhoA activity through its cytoplasmic domain, we measured the activation state of RhoA in hFbs expressing WT-PDPN. RhoA activity was 2.7 fold higher in WT-PDPN expressing hFbs than in control hFbs. Furthermore, the subcutaneous co-injection of hFbs expressing constitutive active RhoA (G14VRhoA) and A549 cells enhanced tumor formation compared with the co-injection of the same cell line and control hFbs. These results indicate that enhanced RhoA activity in hFbs expressing PDPN may be one of the mechanisms resulting in the promotion of tumor formation, suggesting that biomechanical remodeling of the microenvironment by stromal fibroblasts may play important roles in tumor progression.
☆我々の論文4報(Kato JBC 2003; Kato Tumor Biol 2005; Kunita AJP 2007; Suzuki-Inoue JBC 2007)が引用されている。
☆抗体は18H5 (Abcam)が使用されている。
Exp Lung Res. 2012 Jun;38(5):266-76.
Differential alveolar epithelial injury and protein expression in pneumococcal pneumonia.
Tyrrell C, McKechnie SR, Beers MF, Mitchell TJ, McElroy MC.
1MRC Centre for Inflammation Research, Queen's Medical Research Institute, University of Edinburgh, Edinburgh, Scotland, UK.
Abstract
ABSTRACT The integrity of the alveolar epithelium is a key factor in the outcome of acute lung injury. Here, we investigate alveolar epithelial injury and the expression of epithelial-selective markers in Streptococcus pneumoniae-induced acute lung injury. S. pneumoniae was instilled into rat lungs and alveolar type I (RTI(40)/podoplanin, MMC6 antigen) and alveolar type II (MMC4 antigen, surfactant protein D, pro-surfactant protein C, RTII(70)) cell markers were quantified in lavage fluid and lung tissue at 24 and 72 hours. The alveolar epithelium was also examined using electron, confocal, and light microscopy. S. pneumoniae induced an acute inflammatory response as assessed by increased total protein, SP-D, and neutrophils in lavage fluid. Biochemical and morphological studies demonstrated morphologic injury to type II cells but not type I cells. In particular, the expression of RTI(40)/podoplanin was dramatically reduced, on the surface of type I cells, in the absence of morphologic injury. These data demonstrate that type II cell damage can occur in the absence of type I cell injury without affecting the ability of the lung to return to a normal morphology. These data also demonstrate that RTI(40)/podoplanin is not a type I cell phenotypic marker in experimental acute lung injury caused by S. pneumoniae. Given that RTI(40)/podoplanin is an endogenous ligand for the C-type lectin receptor and this receptor plays a role in platelet aggregation and neutrophil activation, we hypothesize that the reduction of RTI(40)/podoplanin on type I cells might be important for the regulation of platelet and/or neutrophil function in experimental acute lung injury.
J Biol Chem. 2012 May 3. [Epub ahead of print]
Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells.
Osada M, Inoue O, Ding G, Shirai T, Ichise H, Hirayama K, Takano K, Yatomi Y, Hirashima M, Fujii H, Suzuki-Inoue K, Ozaki Y.
University of Yamanashi, Japan
Abstract
The platelet activation receptor CLEC-2 plays crucial roles in thrombosis/hemostasis, tumor metastasis, and lymphangiogenesis, although its role in thrombosis/hemostasis remains controversial. An endogenous ligand for CLEC-2, podoplanin, is expressed in lymphatic endothelial cells (LECs). We and others have reported that CLEC-2-deficiency is lethal at mouse embryonic/neonatal stages associated with blood-filled lymphatics, indicating that CLEC-2 is essential for blood/lymphatic vessel separation. However, its mechanism, and whether CLEC-2 in platelets is necessary for this separation, remains unknown. We found that specific deletion of CLEC-2 from platelets leads to the misconnection of blood/lymphatic vessels. CLEC-2+/+ platelets, but not by CLEC-2-/- platelets, inhibited LEC migration, proliferation and tube formation but had no effect on human umbilical vein endothelial cells. Additionally, supernatants from activated platelets significantly inhibited these three functions in LECs, suggesting that released granule contents regulate blood/lymphatic vessel separation. Bone morphologic protein-9 (BMP-9), which we found to be present in platelets and released upon activation, appears to play a key role in regulating LEC functions. Only BMP-9 inhibited tube formation, although other releasates including transforming growth factor β and platelet factor 4 inhibited proliferation and/or migration. We propose that platelets regulate blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of LECs mainly owing to BMP-9 released upon activation by CLEC-2/podoplanin interaction.
福岡歯科大学の沢先生との共同研究の論文が、Acta Histochem. Cytochem.にアクセプトされました。抗ポドプラニン抗体2種類を山形大学で作製し、その解析を沢先生のラボで行って頂きました。
Chiaki Kaj, Yuta Tsujimoto (contributed equally), Mika Kato Kaneko, Yukinari Kato, and Yoshihiko Sawa.
Immunohistochemical Examination of Novel Rat Monoclonal Antibodies against Mouse and Human Podoplanin.
Acta Histochm. Cytochem., in press
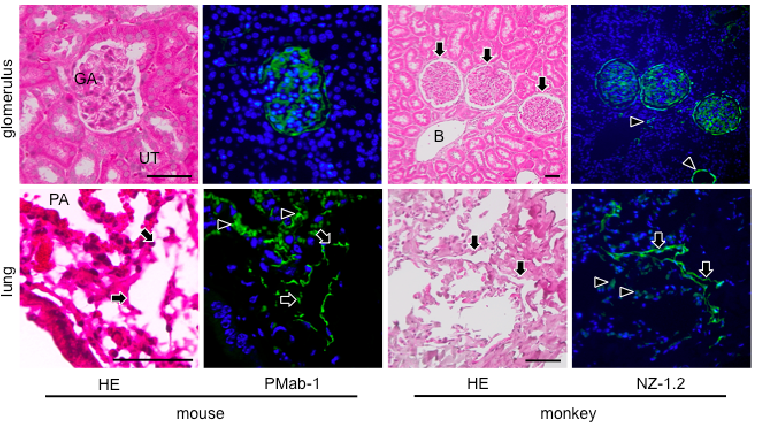
*Wakoから各種抗ポドプラニン抗体が販売になりました。
Ann Surg Oncol. 2012 Jun 6. [Epub ahead of print]
Recruitment of Podoplanin Positive Cancer-Associated Fibroblasts in Metastatic Lymph Nodes Predicts Poor Prognosis in Pathological N2 Stage III Lung Adenocarcinoma.
Neri S, Ishii G, Taira T, Hishida T, Yoshida J, Nishimura M, Nagai K, Ochiai A.
Pathology Division, Research Center for Innovative Oncology, National Cancer Center Hospital East, Kashiwa, Chiba, Japan.
Abstract
BACKGROUND: Cancer-associated fibroblasts (CAFs) directly communicate with cancer cells and play important roles in cancer progression. Recent studies have reported that primary cancer tissue with podoplanin-expressing CAFs predicted a poorer outcome among stage I lung adenocarcinoma patients. However, whether podoplanin(+)-CAFs also can be recruited into metastatic lymph nodes and influence the prognosis remains unclear. METHODS: We selected 112 patients with pathological N2 stage III lung adenocarcinoma and examined the podoplanin expression of CAFs and their prognostic impact in primary and metastatic N2 lesions. RESULTS: Podoplanin(+)-CAFs were observed in 61 (54.5 %) primary sites and 44 (39.3 %) metastatic lymph nodes. Podoplanin(+)-CAFs were found at metastatic lymph nodes in 33 (54.1 %) primary podoplanin-positive and 11 (21.6 %) primary podoplanin-negative sites. These findings suggest a significant positive correlation in podoplanin expression in CAFs between pairs of primary and metastatic lesions (P < 0.001). The difference in the overall survival of patients with podoplanin-positive/negative CAFs in their primary lesion was not correlated (P = 0.927). In contrast, patients with podoplanin(+)-CAFs in metastatic lymph nodes had a shorter overall survival than those without podoplanin(+)-CAFs (P = 0.003). In multivariate analyses, podoplanin(+)-CAFs in metastatic lymph nodes were a significantly independent risk factor for a poor outcome (P = 0.007). CONCLUSIONS: Our study indicated that podoplanin(+)-CAFs in metastatic lymph nodes was a significant prognostic factor for overall survival among pathological N2 stage III adenocarcinoma patients.
PLoS One. 2012;7(7):e41845. Epub 2012 Jul 23.
http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0041845#cor1
Plant lectin can target receptors containing sialic Acid, exemplified by podoplanin, to inhibit transformed cell growth and migration.
Ochoa-Alvarez JA, Krishnan H, Shen Y, Acharya NK, Han M, McNulty DE, Hasegawa H, Hyodo T, Senga T, Geng JG, Kosciuk M, Shin SS, Goydos JS, Temiakov D, Nagele RG, Goldberg GS.
Graduate School of Biomedical Sciences, University of Medicine and Dentistry of New Jersey, Stratford, New Jersey, United States of America.
Abstract
Cancer is a leading cause of death of men and women worldwide. Tumor cell motility contributes to metastatic invasion that causes the vast majority of cancer deaths. Extracellular receptors modified by α2,3-sialic acids that promote this motility can serve as ideal chemotherapeutic targets. For example, the extracellular domain of the mucin receptor podoplanin (PDPN) is highly O-glycosylated with α2,3-sialic acid linked to galactose. PDPN is activated by endogenous ligands to induce tumor cell motility and metastasis. Dietary lectins that target proteins containing α2,3-sialic acid inhibit tumor cell growth. However, anti-cancer lectins that have been examined thus far target receptors that have not been identified. We report here that a lectin from the seeds of Maackia amurensis (MASL) with affinity for O-linked carbohydrate chains containing sialic acid targets PDPN to inhibit transformed cell growth and motility at nanomolar concentrations. Interestingly, the biological activity of this lectin survives gastrointestinal proteolysis and enters the cardiovascular system to inhibit melanoma cell growth, migration, and tumorigenesis. These studies demonstrate how lectins may be used to help develop dietary agents that target specific receptors to combat malignant cell growth.
*NZ-1/NZ-8に関する論文がPubMedに掲載されました。
Kaneko MK, Kunita A, Abe S, Tsujimoto Y, Fukayama M, Goto K, Sawa Y, Nishioka Y, Kato Y.
A chimeric anti-podoplanin antibody suppresses tumor metastasis via neutralization and antibody-dependent cellular cytotoxicity
Cancer Sci. 2012 Jul 20. doi: 10.1111/j.1349-7006.2012.02385.x. [Epub ahead of print]
*ポドプラニンの総説を和光純薬時報に書きました。(和光純薬時報 Vol.80, No.3(2012))
*NZ-1/NZ-8に関する論文がアクセプトされました。ラボとして13報目です。
Kaneko MK, Kunita A, Abe S, Tsujimoto Y, Fukayama M, Goto K, Sawa Y, Nishioka Y, Kato Y.
A chimeric anti-podoplanin antibody suppresses tumor metastasis via neutralization and antibody-dependent cellular cytotoxicity
Cancer Sci., in press
ポドプラニンは、悪性脳腫瘍、中皮腫、各種扁平上皮癌に高発現し、がん細胞の浸潤能や転移能を亢進する。一方、ラット抗ヒトポドプラニン抗体NZ-1は、ポドプラニンによる血小板凝集や実験的転移を抑制する。ヒトキメラ型抗ポドプラニン抗体が、ポドプラニン発現細胞の転移抑制効果があるかどうかを調べ、その作用機序を検討した。
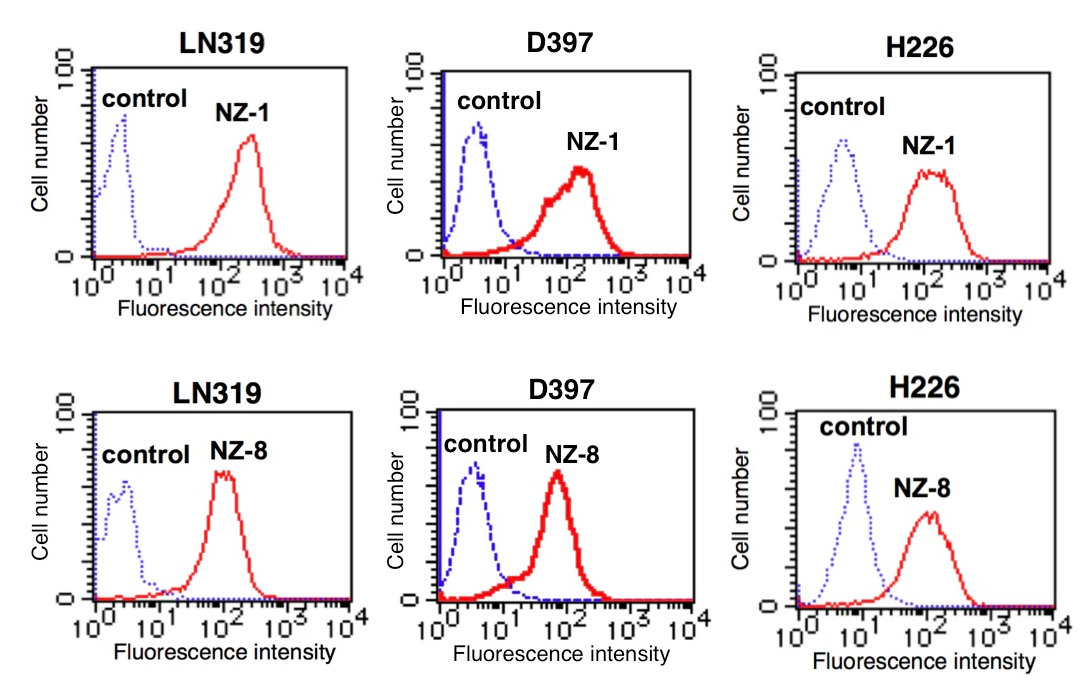
まず、ヒトキメラ型抗ポドプラニン抗体(NZ-8)を作製し、各種がん細胞株に対する反応性を、Flow cytometry法やWestern-blot法により確認した。下の図に示す通り、NZ-8もNZ-1同様に高い反応性を示した。NZ-8はポドプラニンによる血小板凝集を抑制し、転移も有意に抑制した。
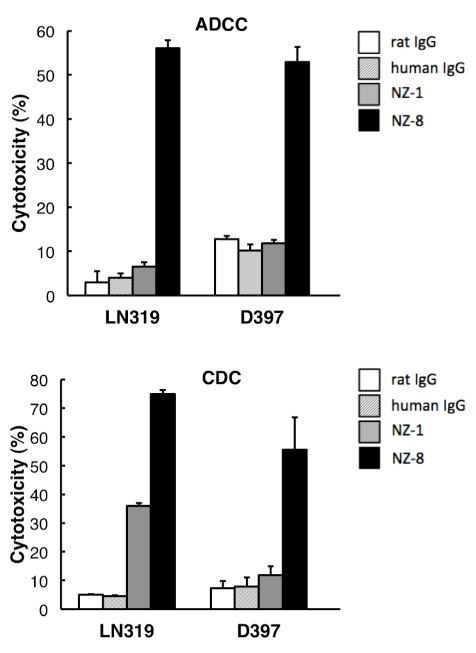
また、抗ポドプラニン抗体の抗体依存性細胞障害活性(ADCC)、補体依存性細胞障害活性(CDC)を検討した。その結果、NZ-1と比較して、NZ-8はポドプラニン発現株に対し高いADCC活性、CDC活性を示した。
一方、NZ-8はin vivoでのポドプラニン発現株の増殖を有意に抑制した。
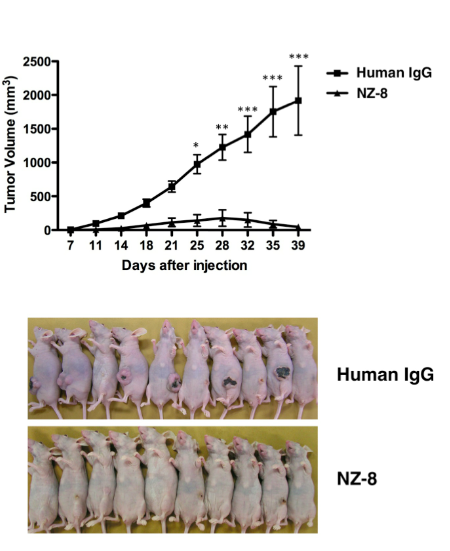
以上の結果より、ヒトキメラ型抗ポドプラニン抗体は、血小板凝集阻害活性だけでなく、腫瘍細胞に対して高いエフェクター活性を示すことにより、がん転移を抑制することが示唆された。一方、一般的に転移をおこさない悪性脳腫瘍や悪性中皮腫に対する抗体医薬として、NZ-8が有用である可能性も示された。
Immunity. 2012 Aug 9. [Epub ahead of print]
Podoplanin-Rich Stromal Networks Induce Dendritic Cell Motility via Activation of the C-type Lectin Receptor CLEC-2.
Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, Bellemare-Pelletier A, Sceats L, Reynoso ED, Gonzalez SF, Graham DB, Chang J, Peters A, Woodruff M, Kim YA, Swat W, Morita T, Kuchroo V, Carroll MC, Kahn ML, Wucherpfennig KW, Turley SJ.
Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, Boston, MA 02215, USA; Department of Cell and Developmental Biology, University College London, London WC1E 6BT, UK.
Abstract
To initiate adaptive immunity, dendritic cells (DCs) move from parenchymal tissues to lymphoid organs by migrating along stromal scaffolds that display the glycoprotein podoplanin (PDPN). PDPN is expressed by lymphatic endothelial and fibroblastic reticular cells and promotes blood-lymph separation during development by activating the C-type lectin receptor, CLEC-2, on platelets. Here, we describe a role for CLEC-2 in the morphodynamic behavior and motility of DCs. CLEC-2 deficiency in DCs impaired their entry into lymphatics and trafficking to and within lymph nodes, thereby reducing T cell priming. CLEC-2 engagement of PDPN was necessary for DCs to spread and migrate along stromal surfaces and sufficient to induce membrane protrusions. CLEC-2 activation triggered cell spreading via downregulation of RhoA activity and myosin light-chain phosphorylation and triggered F-actin-rich protrusions via Vav signaling and Rac1 activation. Thus, activation of CLEC-2 by PDPN rearranges the actin cytoskeleton in DCs to promote efficient motility along stromal surfaces.
1. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos.
Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Blood. 2012 Aug 2. [Epub ahead of print]
PMID: 22859612 [PubMed - as supplied by publisher]
2. Functional invadopodia formation through stabilization of the PDPN transcript by IMP-3 and cancer-stromal crosstalk for PDPN expression.
Hwang YS, Zhang X, Park KK, Chung WY.
Carcinogenesis. 2012 Aug 1. [Epub ahead of print]
PMID: 22859271 [PubMed - as supplied by publisher]
Blood. 2012 Oct 10. [Epub ahead of print]
A detailed proteomic analysis of rhodocytin-activated platelets reveals novel clues on the CLEC-2 signalosome: implications for CLEC-2 signaling regulation.
Parguina AF, Alonso J, Rosa I, Velez P, Gonzalez-Lopez MJ, Guitian E, Ebble JA, Loza MI, Garcia A.
Center for Research in Molecular Medicine and Chronic Diseases (CIMUS), Universidade de Santiago de Compostela, and Instituto de Investigacion Sanitaria de Santiago (IDIS), Santiago de Compostela, Spain;
Abstract
C-type lectin-like receptor 2 (CLEC-2) is an essential platelet-activating receptor in hemostasis and thrombosis that is activated by the snake venom rhodocytin. We present here a differential proteomic analysis of basal and rhodocytin-activated platelets with the aim of providing novel clues on CLEC-2 signaling regulation. Proteome analysis was based on 2D-DIGE, phospho-tyrosine immunoprecipitations followed by 1D-SDS-PAGE, and mass spectrometry. Protein-protein interactions were studied by co-immunoprecipitations and a systems biology approach. Overall, we identified 132 proteins differentially regulated following CLEC-2 platelet activation, including most of the major players reported so far in the signaling cascade. In addition, we identified various proteins not previously known to participate in CLEC-2 signaling, such as the adapters Dok-2 and ADAP, tyrosine kinase Fer, and tyrosine phosphatase SHIP-1. We also report an increased association between Dok-2 and SHIP-1 in rhodocytin-stimulated platelets, which might negatively regulate CLEC-2 signaling. Moreover, we also present a comparative analysis of proteomic data for CLEC-2 and GPVI signaling. We believe our data provide thrombosis-relevant information on CLEC-2 signaling regulation, contributing to a better understanding of this important signaling cascade.
J Cell Biochem. 2012 Nov 13. doi: 10.1002/jcb.24449. [Epub ahead of print]
CXCL14 enhances proliferation and migration of NCI-H460 human lung cancer cells overexpressing the glycoproteins containing heparan sulfate or sialic acid.
Park CR, You DJ, Kim DK, Moon MJ, Lee C, Oh SH, Ahn C, Seong JY, Hwang JI.
Graduate School of Medicine; Korea University, 73 Inchon-ro, Seongbuk-gu, Seoul 136-705, Republic of Korea.
Abstract
CXCL14 is a chemokine family member that is involved in various cellular responses in addition to immune cell activation. Although constitutive CXCL14 expression in normal epithelial cells may help protect against infection by activating immune systems, its expression in cancer cells has raised controversy regarding its possible role in tumorigenesis. However, the underlying mechanisms for this disparity remain unknown. Investigation of cellular CXCL14 binding properties might increase our understanding of the peptide's roles in tumorigenesis. In the present study, we found that CXCL14 binds to various cell types. Interestingly, binding to NCI-H460 cells was prevented by heparan sulfate and N-acetyl neuraminic acid. Next, we examined effect of CXCL14 binding in NCI-H460 and NCI-H23. CXCL14 enhanced proliferation and migration in NCI-H460 but had no effect on NCI-H23. A reporter gene assay with various transcription factor response elements revealed that only nuclear factor-κB (NF-κB) signaling was activated by CXCL14 in NCI-H460 cells, which was blocked by BAPTA-AM, TCPA, and brefeldin A. Exogenous expression of some glycoproteins such as syndecan-4, podoplanin, and CD43 in these cells enhanced CXCL14 binding and NF-κB activity. Collectively, these results demonstrate that CXCL14 binding to glycoproteins harboring heparan sulfate proteoglycans and sialic acids leads proliferation and migration of some cancer cells
*Duke大学からの論文がon lineに掲載されました(IJC)。加藤が作製した抗ポドプラニン抗体(NZ-1)をDuke大学に持って行き、金子がscFvに改変後、インド人のポスドクが活性評価をした仕事です。近々、Duke大学にて、Phase Iが行われることになると思います。(PDF)

この抗体は、加藤が日本で作製した(made in Japan)のですが、このように開発は欧米で先に行われるというのが残念なことです。このNZ-1-scFvの特許もDuke大学から出されていますが、そこには我々日本人の名前が入っておらず、つまり権利はすべてDuke大学に持っていかれた、ということになります。ただ、NZ-1自体の特許は、2年前に日本から出しており、scFvも請求項に入っています(PCT出願)。日本での開発を急がなくてはなりませんが、何しろ開発費がない(企業からも国からも)というのが問題です。自分の開発した薬(抗体医薬)が患者さんの病気を治すことが私の研究の最終目標ですので、開発の場が日本であろうとアメリカであろうと、あるいは自分が最後まで関わるかどうかは関係ないかもしれません。自分のできる仕事を進めるだけです。
Cell Signal. 2012 Dec 21. pii: S0898-6568(12)00341-5. doi: 10.1016/j.cellsig.2012.12.004. [Epub ahead of print]
Intercellular contact augments epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 3 (STAT3)-activation which increases podoplanin-expression in order to promote squamous cell carcinoma motility.
Fujii M, Honma M, Takahashi H, Ishida-Yamamoto A, Iizuka H.
Department of Dermatology, Asahikawa Medical University, Midorigaoka-Higashi 2-1-1-1, Asahikawa, Japan. Electronic address: ma0dj@asahikawa-med.ac.jp.
Abstract
The transmembrane glycoprotein podoplanin (PDPN) plays an important role in cell motility. However, mechanisms regulating PDPN expression have not been fully elucidated. Here, we investigated the effect of intercellular contact on signal transduction pathways and PDPN expression in human squamous cell carcinoma (SCC) cell lines. PDPN expression was higher in confluent SCC cells than sparse cultures. This PDPN induction leads to increased SCC cell migration and invasion, which was reversed by shRNA PDPN knockdown. This cell density dependent PDPN induction required activation of epidermal growth factor receptor (EGFR) and its effector, signal transducer and activator of transcription 3 (STAT3). These observations also extend to human clinical specimens, in which PDPN expression localized to confluent basal cell layers at the invading front of in situ SCC lesions. Taken together, these results illuminate an EGFR-STAT3-PDPN pathway as a potential pharmacological opportunity to target invasive SCC cells.
Int J Biochem Cell Biol. 2012 Dec 11. pii: S1357-2725(12)00400-1. doi: 10.1016/j.biocel.2012.12.004. [Epub ahead of print]
Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin.
Sun Q, Zhang J, Cao W, Wang X, Xu Q, Yan M, Wu X, Chen W.
Department of Oral and Maxillofacial-Head & Neck Oncology, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China; Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology, Shanghai 200011, China.
Abstract
Head and neck squamous cell carcinoma (HNSCC) is characterised by an elevated capacity for tumor invasion and lymph node metastasis and the cause remains to be determined. Recent studies suggest that microRNAs can regulate the evolution of malignant behaviours by regulating multiple target genes. In this study, we have first confirmed that miR-363 is down-regulated in HNSCC tissues with lymph node metastasis and cell lines with highly invasive capacity. We used bioinformatics, cellular and molecular methods to predict and prove that miR-363 directly targeted to podoplanin (PDPN) and caused up-regulation of PDPN in HNSCC. MSP assay showed that DNA promoter methylation was involved in silencing the miR-363 in HNSCC. Furthermore, we provided evidence to demonstrate that PDPN dysregulation caused by down-regulation of miR-363 contributes to HNSCC invasion and metastasis. These data reveal a key role of miR-363-PDPN in HNSCC metastasis and support biological and clinical links between miR-363-PDPN and HNSCC.
発明の名称 : マウス抗Aggrusモノクローナル抗体(PCT出願)
[0001]
本願発明は、マウス抗Aggrusモノクローナル抗体に関する。
背景技術
[0002]
血小板凝集誘導因子Aggrus(podoplanin、gp44等としても知られる)は、I型膜貫通タンパク質であり、扁平上皮癌、中皮腫、カポジ肉腫、精巣腫瘍及び脳腫瘍といった様々なタイプの癌で発現増加していることが示されている(非特許文献1~9)。Aggrusの過剰発現は予後不良に関係するとの報告があり、癌進行に対するAggrusの重要な関与が示唆されている(非特許文献10、11)。Aggrusの発現が血小板凝集を引き起こし、マウスにおける実験的な、そして自発的な肺転移を促進することが知られている(非特許文献11、12)。血小板凝集を抑制する点突然変異を導入すると、肺転移の形成は減弱するので、Aggrusの血小板凝集誘導活性は転移形成に直接関係していると考えられている(非特許文献11、12)。癌細胞誘導血小板凝集は、大きな癌-血小板凝集を形成し、微小血管系での癌細胞の塞栓形成増大、循環での免疫学的攻撃からの保護に至ると考えられている。最近、血小板上に発現しているC型レクチン様受容体(CLEC-2)が、Aggrusのカウンターパート受容体の一つとして特定された。腫瘍細胞上で発現しているAggrusにCLEC-2が結合すると、血漿成分がなくとも血小板で活性化シグナルが出され、血小板凝集の引き金となることが知られている。相互認識に決定的なAggrus及びCLEC-2のドメインは、すでに知られている(非特許文献13)。
[0003]
モノクローナル抗体は、細胞表面抗原にしっかりと特異的に結合することができ、標的細胞に免疫学的応答を引き起こすことができる。従って、現在、多くのモノクローナル抗体が癌治療に用いられている。癌治療で用いられるモノクローナル抗体は、中和、抗体依存性細胞傷害活性(antibody-dependent cellular cytotoxicity;ADCC)及び補体依存性細胞傷害活性(complement-dependent cytotoxicity;CDC)、の3つの代表的な作用様式を介して抗癌効果を示す。ベバシズマブやセツキシマブに代表されるように、抗体はリガンド-受容体結合又は受容体多量体化を阻害し、シグナル経路の活性化を中和することができる。癌細胞の増殖はシグナル経路の活性化に依存しているので、その中和により細胞死に至る。他方、リツキシマブやトラスツズマブに代表されるように、抗体はそのFcドメインを介して標的癌細胞に対する免疫応答を誘導することができる。マクロファージ、NK細胞及び好中球等のエフェクター細胞と補体は、癌特異的抗原に結合する抗体のFcドメインを認識し、その結果標的癌細胞を殺す。これらの3つの作用様式は、抗体のアイソタイプやサブクラス、抗原の特徴や認識される部位により規定される。
[0004]
これまで、Aggrusに対する多くの抗体が確立されてきたが、そのほとんどはAggrus-CLEC-2の相互作用を干渉できないものであった。NZ-1と命名されたラット抗体は、Aggrus-CLEC-2の相互作用および血小板凝集を阻害することができるAggrus抗体として知られているが(非特許文献14)、種のバリアのせいで一般的に用いられるマウスの癌モデルでは正確に調べることができない等の問題点があった。